Acute Crit Care.
2020 Nov;35(4):255-262. 10.4266/acc.2020.00164.
Utilization of pain and sedation therapy on noninvasive mechanical ventilation in Korean intensive care units: a multi-center prospective observational study
- Affiliations
-
- 1Department of Pulmonary, Allergy and Critical Care Medicine, Hallym University Kangnam Sacred Heart Hospital, Seoul, Korea
- 2Department of Pulmonary and Critical Care Medicine, Inha University College of Medicine, Incheon, Korea
- 3Department of Pulmonary and Critical Care Medicine, Yeungnam University Hospital, Daegu, Korea
- 4Department of Pulmonary and Critical Care Medicine, Inje University Sanggye Paik Hospital, Seoul, Korea
- 5Department of Internal Medicine, Myongji Hospital, Goyang, Korea
- 6Department of Pulmonary and Critical Care Medicine, Kyung Hee University Hospital at Gangdong, Seoul, Korea
- 7Department of Pulmonary and Critical Care Medicine, Chungnam National University Hospital, Daejeon, Korea
- 8Department of Internal Medicine, Pusan National University Hospital, Busan, Korea
- 9Department of Pulmonary, Critical Care and Sleep Medicine, St. Paul’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 10Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Inje University Ilsan Paik Hospital, Goyang, Korea
- 11Department of Internal Medicine, Hanyang University Guri Hospital, Guri, Korea
- 12Department of Pulmonary, Allergy and Critical Care Medicine, Jeonbuk National University Hospital, Jeonju, Korea
- 13Department of Pulmonary, Allergy and Critical Care Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea
- 14Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2510544
- DOI: http://doi.org/10.4266/acc.2020.00164
Abstract
- Background
The use of sedative drugs may be an important therapeutic intervention during noninvasive ventilation (NIV) in intensive care units (ICUs). The purpose of this study was to assess the current application of analgosedation in NIV and its impact on clinical outcomes in Korean ICUs.
Methods
Twenty Korean ICUs participated in the study, and data was collected on NIV use during the period between June 2017 and February 2018. Demographic data from all adult patients, NIV clinical parameters, and hospital mortality were included.
Results
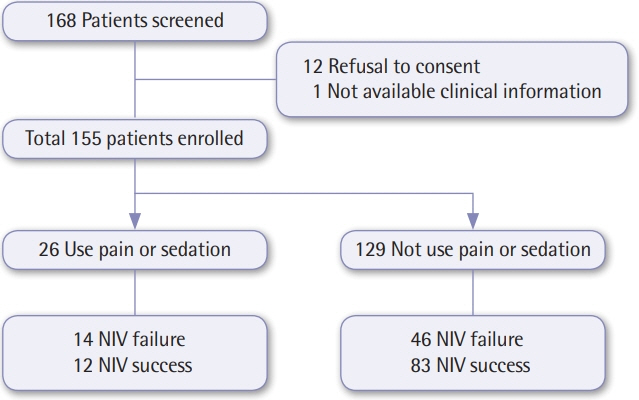
A total of 155 patients treated with NIV in the ICUs were included, of whom 26 received pain and sedation therapy (sedation group) and 129 did not (control group). The primary cause of ICU admission was due to acute exacerbation of obstructed lung disease (45.7%) in the control group and pneumonia treatment (53.8%) in the sedation group. In addition, causes of NIV application included acute hypercapnic respiratory failure in the control group (62.8%) and post-extubation respiratory failure in the sedation group (57.7%). Arterial partial pressure of carbon dioxide (PaCO2) levels before and after 2 hours of NIV treatment were significantly decreased in both groups: from 61.9±23.8 mm Hg to 54.9±17.6 mm Hg in the control group (P<0.001) and from 54.9±15.1 mm Hg to 51.1±15.1 mm Hg in the sedation group (P=0.048). No significant differences were observed in the success rate of NIV weaning, complications, length of ICU stay, ICU survival rate, or hospital survival rate between the groups.
Conclusions
In NIV patients, analgosedation therapy may have no harmful effects on complications, NIV weaning success, and mortality compared to the control group. Therefore, sedation during NIV may not be unsafe and can be used in patients for pain control when indicated.
Figure
Cited by 1 articles
-
2021 KSCCM clinical practice guidelines for pain, agitation, delirium, immobility, and sleep disturbance in the intensive care unit
Yijun Seo, Hak-Jae Lee, Eun Jin Ha, Tae Sun Ha
Acute Crit Care. 2022;37(1):1-25. doi: 10.4266/acc.2022.00094.
Reference
-
1. Walkey AJ, Wiener RS. Use of noninvasive ventilation in patients with acute respiratory failure, 2000-2009: a population-based study. Ann Am Thorac Soc. 2013; 10:10–7.2. Girou E, Brun-Buisson C, Taillé S, Lemaire F, Brochard L. Secular trends in nosocomial infections and mortality associated with noninvasive ventilation in patients with exacerbation of COPD and pulmonary edema. JAMA. 2003; 290:2985–91.
Article3. Hilbert G, Navalesi P, Girault C. Is sedation safe and beneficial in patients receiving NIV? Yes. Intensive Care Med. 2015; 41:1688–91.
Article4. Carron M, Freo U, BaHammam AS, Dellweg D, Guarracino F, Cosentini R, et al. Complications of non-invasive ventilation techniques: a comprehensive qualitative review of randomized trials. Br J Anaesth. 2013; 110:896–914.
Article5. Conti G, Arcangeli A, Antonelli M, Cavaliere F, Costa R, Simeoni F, et al. Sedation with sufentanil in patients receiving pressure support ventilation has no effects on respiration: a pilot study. Can J Anaesth. 2004; 51:494–9.
Article6. Ethuin F, Boudaoud S, Leblanc I, Troje C, Marie O, Levron JC, et al. Pharmacokinetics of long-term sufentanil infusion for sedation in ICU patients. Intensive Care Med. 2003; 29:1916–20.
Article7. Constantin JM, Schneider E, Cayot-Constantin S, Guerin R, Bannier F, Futier E, et al. Remifentanil-based sedation to treat noninvasive ventilation failure: a preliminary study. Intensive Care Med. 2007; 33:82–7.8. Akada S, Takeda S, Yoshida Y, Nakazato K, Mori M, Hongo T, et al. The efficacy of dexmedetomidine in patients with noninvasive ventilation: a preliminary study. Anesth Analg. 2008; 107:167–70.
Article9. Clouzeau B, Bui HN, Vargas F, Grenouillet-Delacre M, Guilhon E, Gruson D, et al. Target-controlled infusion of propofol for sedation in patients with non-invasive ventilation failure due to low tolerance: a preliminary study. Intensive Care Med. 2010; 36:1675–80.
Article10. Rocco M, Conti G, Alessandri E, Morelli A, Spadetta G, Laderchi A, et al. Rescue treatment for noninvasive ventilation failure due to interface intolerance with remifentanil analgosedation: a pilot study. Intensive Care Med. 2010; 36:2060–5.
Article11. Huang Z, Chen YS, Yang ZL, Liu JY. Dexmedetomidine versus midazolam for the sedation of patients with non-invasive ventilation failure. Intern Med. 2012; 51:2299–305.
Article12. Nam H, Cho JH, Choi EY, Chang Y, Choi WI, Hwang JJ, et al. Current status of noninvasive ventilation use in Korean intensive care units: a prospective multicenter observational study. Tuberc Respir Dis (Seoul). 2019; 82:242–50.
Article13. Demoule A, Chevret S, Carlucci A, Kouatchet A, Jaber S, Meziani F, et al. Changing use of noninvasive ventilation in critically ill patients: trends over 15 years in francophone countries. Intensive Care Med. 2016; 42:82–92.14. Demoule A, Girou E, Richard JC, Taillé S, Brochard L. Increased use of noninvasive ventilation in French intensive care units. Intensive Care Med. 2006; 32:1747–55.
Article15. Shaheen M, Daabis RG, Elsoucy H. Outcomes and predictors of success of noninvasive ventilation in acute exacerbation of chronic obstructive pulmonary disease. Egypt Bronchology. 2018; 12:329–39.
Article16. Moretti M, Cilione C, Tampieri A, Fracchia C, Marchioni A, Nava S. Incidence and causes of non-invasive mechanical ventilation failure after initial success. Thorax. 2000; 55:819–25.
Article17. Soo Hoo GW, Santiago S, Williams AJ. Nasal mechanical ventilation for hypercapnic respiratory failure in chronic obstructive pulmonary disease: determinants of success and failure. Crit Care Med. 1994; 22:1253–61.18. Devlin JW, Nava S, Fong JJ, Bahhady I, Hill NS. Survey of sedation practices during noninvasive positive-pressure ventilation to treat acute respiratory failure. Crit Care Med. 2007; 35:2298–302.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multicenter Prospective Observational Study about the Usage Patterns of Sedatives, Analgesics and Neuromuscular Blocking Agents in the Patients Requiring More Than 72 Hours Mechanical Ventilation in Intensive Care Units of Korea
- Erratum to “Utilization of pain and sedation therapy on noninvasive mechanical ventilation in Korean intensive care units: a multi-center prospective observational study”
- Usefulness of Noninvasive Ventilation with Negative-Pressure Wound Therapy in the Intensive Care Unit: A Case Report
- New Modalities and Modes in Neonatal Ventilation Therapy
- Sedation in the Critically Ill Patients