Korean J Transplant.
2020 Dec;34(4):279-285. 10.4285/kjt.20.0017.
Liver transplantation for azithromycin-induced severe liver injury
- Affiliations
-
- 1Department of Internal Medicine, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- 2Department of Surgery, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- KMID: 2510267
- DOI: http://doi.org/10.4285/kjt.20.0017
Abstract
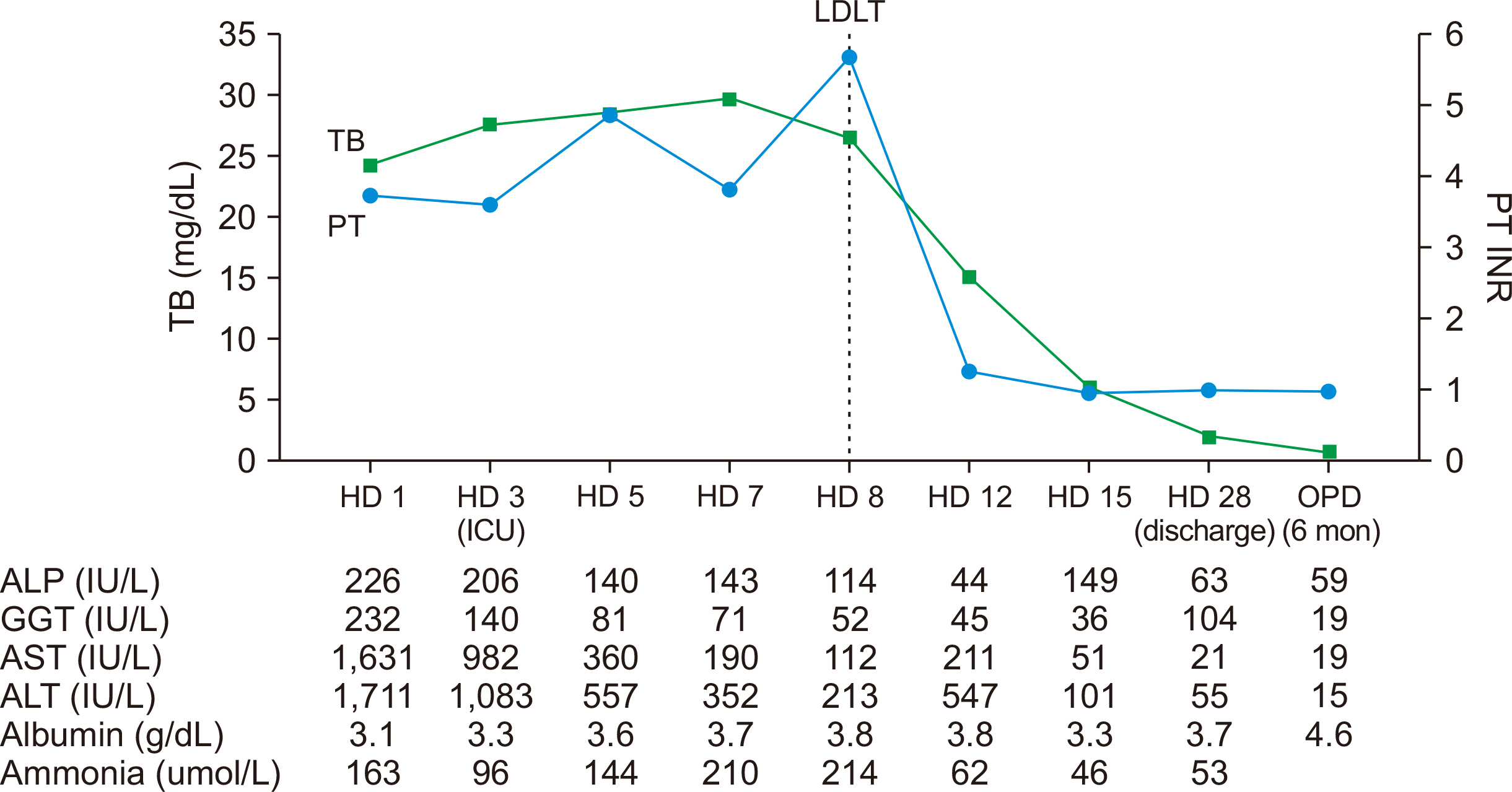
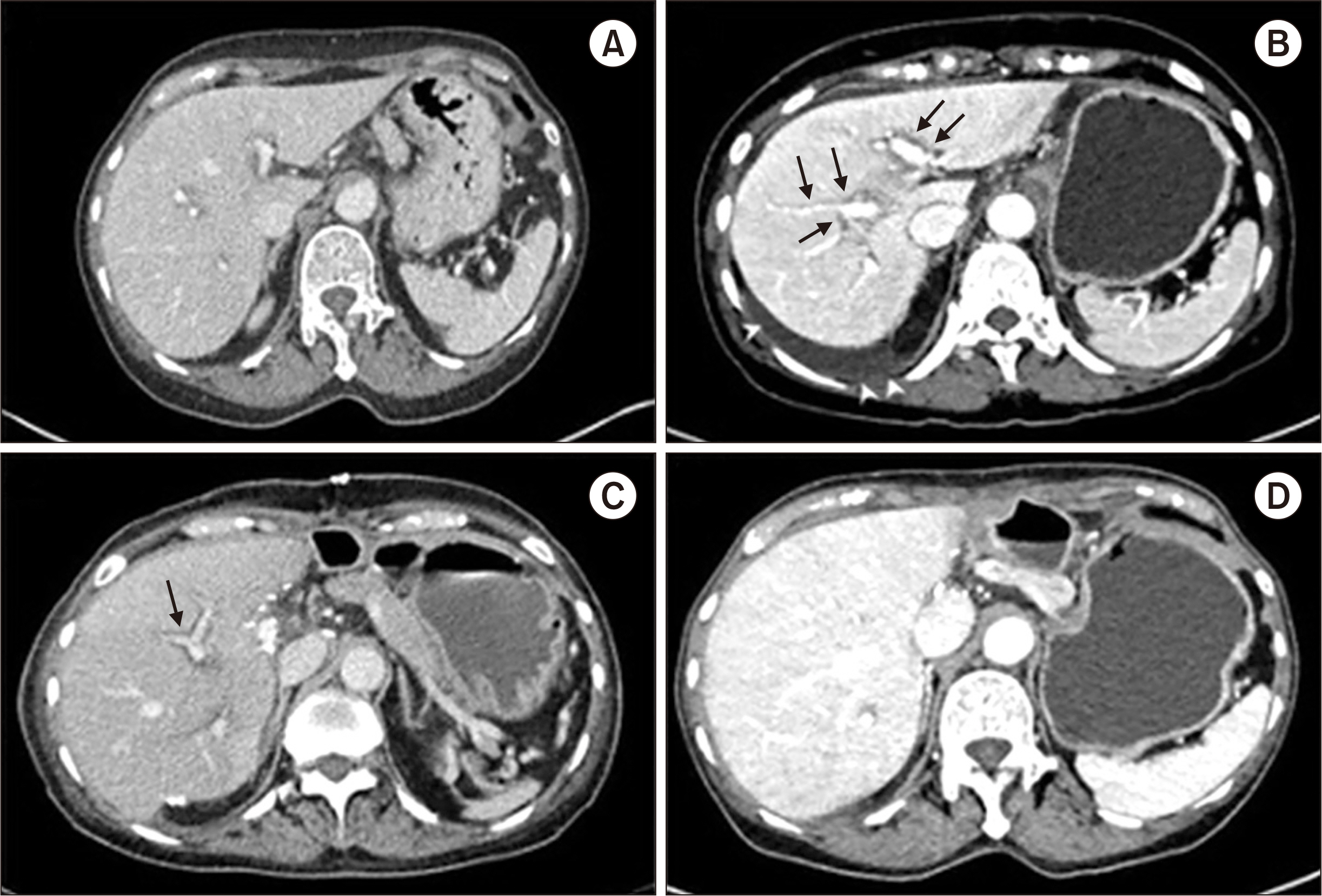
- Drug-induced liver injury is the most common cause of acute liver failure in Western countries by prescription drugs and herbal medications. Liver injury due to azithromycin has rarely been reported. This is a brief report of a patient administered azithromycin and who developed acute liver failure leading to liver transplantation. We report the case of a 68-year-old woman who developed jaundice 1 week after she started taking a azith-romycin. On the 3rd day of hospitalization, her hepatic function rapidly deteriorated and level of consciousness decreased to drowsiness. The model for end-stage liver disease score was confirmed to be 33, and liver transplantation was considered. On the 8th day of hospitalization, she underwent emergency living donor liver transplantation, receiving a right lobe liver graft from a 35-year-old male donor, the patient’s son. Currently, she is alive with good liver function after 25 months of transplant. This case suggests that azithromycin may cause rare hepatitis with liver failure. Therefore, at the beginning of the azithromycin treatment, patients should visit the hospital immediately if symptoms such as jaundice and abdominal pain are experienced.
Figure
Reference
-
1. Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C, et al. 2002; Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 36:451–5. DOI: 10.1053/jhep.2002.34857. PMID: 12143055.
Article2. Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. 2013; Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 144:1419–25. DOI: 10.1053/j.gastro.2013.02.006. PMID: 23419359.
Article3. Vuppalanchi R, Liangpunsakul S, Chalasani N. 2007; Etiology of new-onset jaundice: how often is it caused by idiosyncratic drug-induced liver injury in the United States? Am J Gastroenterol. 102:558–62. DOI: 10.1111/j.1572-0241.2006.01019.x. PMID: 17156142.
Article4. Reuben A, Koch DG. Lee WM; Acute Liver Failure Study Group. 2010; Drug-induced acute liver failure: results of a U. S. multicenter, prospective study. Hepatology. 52:2065–76. DOI: 10.1002/hep.23937. PMID: 20949552. PMCID: PMC3992250.5. Wei G, Bergquist A, Broomé U, Lindgren S, Wallerstedt S, Almer S, et al. 2007; Acute liver failure in Sweden: etiology and outcome. J Intern Med. 262:393–401. DOI: 10.1111/j.1365-2796.2007.01818.x. PMID: 17697161.
Article6. Zuckerman JM, Qamar F, Bono BR. 2009; Macrolides, ketolides, and glycylcyclines: azithromycin, clarithromycin, telithromycin, tigecycline. Infect Dis Clin North Am. 23:997–1026. DOI: 10.1016/j.idc.2009.06.013. PMID: 19909895.
Article7. Hicks LA, Taylor TH Jr, Hunkler RJ. 2013; U.S. outpatient antibiotic prescribing, 2010. N Engl J Med. 368:1461–2. DOI: 10.1056/NEJMc1212055. PMID: 23574140.
Article8. Lee EJ, Park J, Lee GW, Kim DS. 2019; The use of broad-spectrum antibiotics and antibiotics to treat antimicrobial-resistant bacteria. Yakhakhoe Chi. 63:43–53. DOI: 10.17480/psk.2019.63.1.43.
Article9. Principi N, Esposito S. 1999; Comparative tolerability of erythromycin and newer macrolide antibacterials in paediatric patients. Drug Saf. 20:25–41. DOI: 10.2165/00002018-199920010-00004. PMID: 9935275.
Article10. Martinez MA, Vuppalanchi R, Fontana RJ, Stolz A, Kleiner DE, Hayashi PH, et al. 2015; Clinical and histologic features of azithromycin-induced liver injury. Clin Gastroenterol Hepatol. 13:369–76. DOI: 10.1016/j.cgh.2014.07.054. PMID: 25111234. PMCID: PMC4321982.
Article11. Danan G, Teschke R. 2015; RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci. 17:14. DOI: 10.3390/ijms17010014. PMID: 26712744. PMCID: PMC4730261.
Article12. Hoofnagle JH, Björnsson ES. 2019; Drug-induced liver injury: types and phenotypes. N Engl J Med. 381:264–73. DOI: 10.1056/NEJMra1816149. PMID: 31314970.13. Leise MD, Poterucha JJ, Talwalkar JA. 2014; Drug-induced liver injury. Mayo Clin Proc. 89:95–106. DOI: 10.1016/j.mayocp.2013.09.016. PMID: 24388027.
Article14. Kullak-Ublick GA, Andrade RJ, Merz M, End P, Benesic A, Gerbes AL, et al. 2017; Drug-induced liver injury: recent advances in diagnosis and risk assessment. Gut. 66:1154–64. DOI: 10.1136/gutjnl-2016-313369. PMID: 28341748. PMCID: PMC5532458.
Article15. Temple R. 2006; Hy's law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 15:241–3. DOI: 10.1002/pds.1211. PMID: 16552790.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Liver transplantation for azithromycin-induced severe liver injury: a case report
- Pediatric liver transplantation in Korea: long-term outcomes and allocations
- Launching Annals of Liver Transplantation: First small steps toward remarkable academic achievements in liver transplantation
- Successful salvage treatment of acute graft-versus-host disease after liver transplantation by withdrawal of immunosuppression: a case report
- The immunosuppressive effects of liver regeneration factor after 30% and 100% liver transplantation in rat