Korean J Physiol Pharmacol.
2021 Jan;25(1):79-85. 10.4196/kjpp.2021.25.1.79.
Immunohistochemical detection of GluA1 subunit of AMPA receptor in the rat nucleus accumbens following cocaine exposure
- Affiliations
-
- 1Department of Physiology, Graduate School of Medical Sciences, Yonsei University College of Medicine, Seoul 03722, Korea
- KMID: 2509657
- DOI: http://doi.org/10.4196/kjpp.2021.25.1.79
Abstract
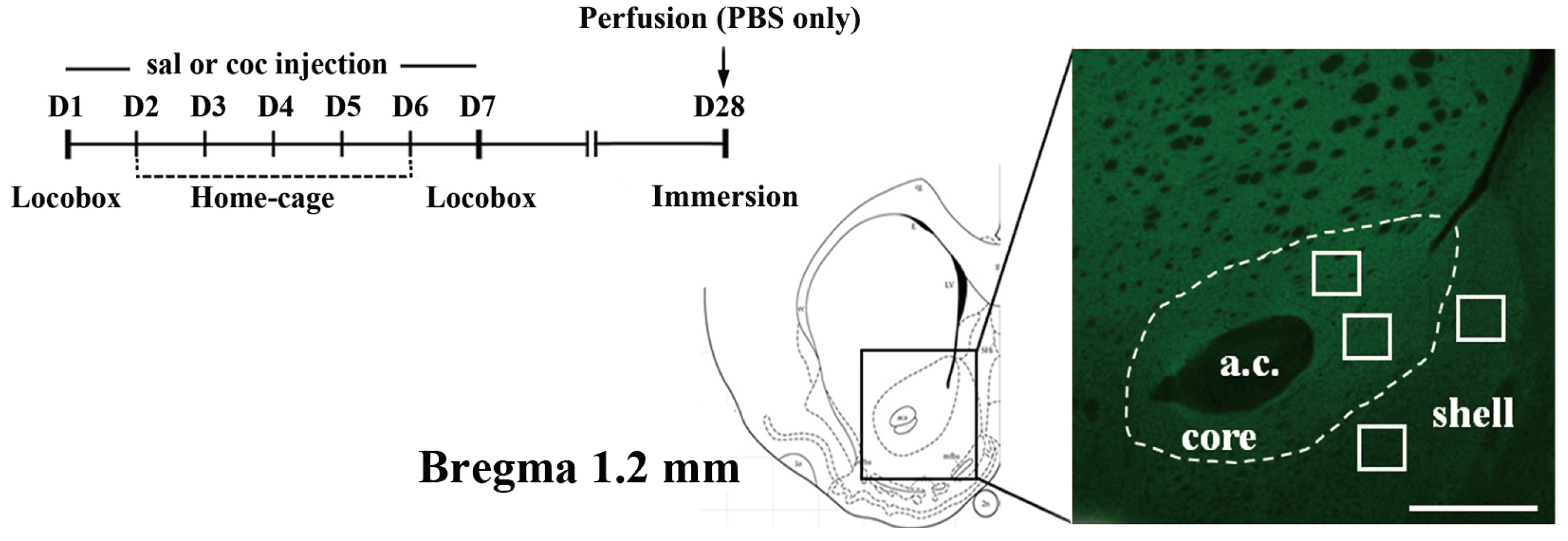
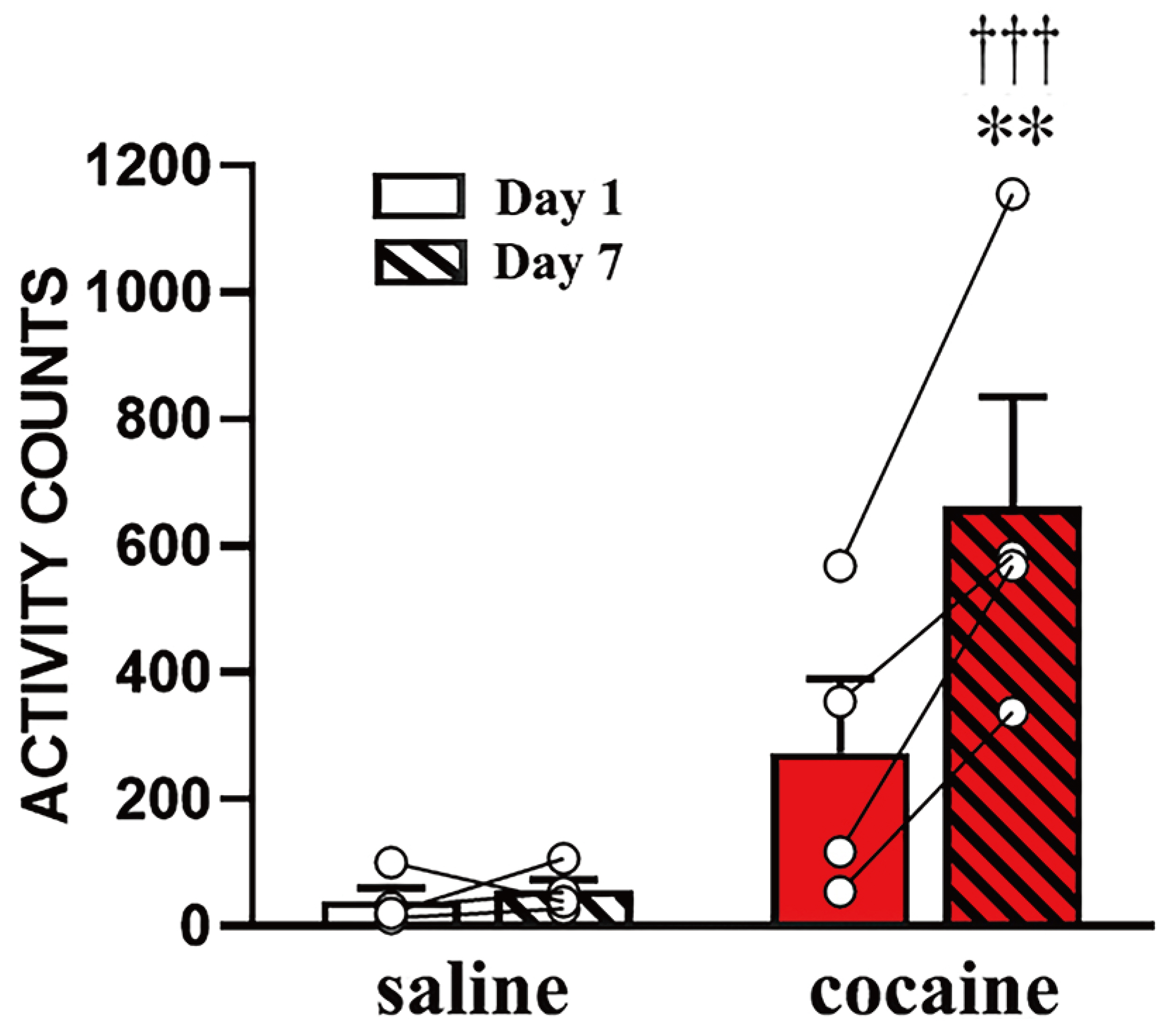
- α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors are differentially regulated in the nucleus accumbens (NAcc) of the brain after cocaine exposure. However, these results are supported only by biochemical and electrophysiological methods, but have not been validated with immunohistochemistry. To overcome the restriction of antigen loss on the postsynaptic target molecules that occurs during perfusion-fixation, we adopted an immersion-fixation method that enabled us to immunohistochemically quantify the expression levels of the AMPA receptor GluA1 subunit in the NAcc. Interestingly, compared to saline exposure, cocaine significantly increased the immunofluorescence intensity of GluA1 in two sub-regions, the core and the shell, of the NAcc on withdrawal day 21 following cocaine exposure, which led to locomotor sensitization. Increases in GluA1 intensity were observed in both the extra-post synaptic density (PSD) and PSD areas in the two sub-regions of the NAcc. These results clearly indicate that AMPA receptor plasticity, as exemplified by GluA1, in the NAcc can be visually detected by immunohistochemistry and confocal imaging. These results expand our understanding of the molecular changes occurring in neuronal synapses by adding a new form of analysis to conventional biochemical and electrophysiological methods.
Figure
Reference
-
1. Kalivas PW, Volkow ND. 2005; The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 162:1403–1413. DOI: 10.1176/appi.ajp.162.8.1403. PMID: 16055761.
Article2. Goto Y, Grace AA. 2008; Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci. 31:552–558. DOI: 10.1016/j.tins.2008.08.002. PMID: 18786735. PMCID: PMC2884964.
Article3. Groenewegen HJ, Wright CI, Beijer AV, Voorn P. 1999; Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 877:49–63. DOI: 10.1111/j.1749-6632.1999.tb09260.x. PMID: 10415642.
Article4. Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Lüscher C. 2009; Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 12:1036–1041. DOI: 10.1038/nn.2367. PMID: 19597494.
Article5. Pierce RC, Wolf ME. 2013; Psychostimulant-induced neuroadaptations in nucleus accumbens AMPA receptor transmission. Cold Spring Harb Perspect Med. 3:a012021. DOI: 10.1101/cshperspect.a012021. PMID: 23232118. PMCID: PMC3552338.
Article6. Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. 2008; Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 454:118–121. DOI: 10.1038/nature06995. PMID: 18500330. PMCID: PMC2574981.
Article7. Wolf ME, Ferrario CR. 2010; AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 35:185–211. DOI: 10.1016/j.neubiorev.2010.01.013. PMID: 20109488. PMCID: PMC2962767.
Article8. Wolf ME, Tseng KY. 2012; Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci. 5:72. DOI: 10.3389/fnmol.2012.00072. PMID: 22754497. PMCID: PMC3384237.
Article9. Pierce RC, Bell K, Duffy P, Kalivas PW. 1996; Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 16:1550–1560. DOI: 10.1523/JNEUROSCI.16-04-01550.1996. PMID: 8778304. PMCID: PMC6578548.
Article10. Bäckström P, Hyytiä P. 2007; Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 192:571–580. DOI: 10.1007/s00213-007-0753-8. PMID: 17347848.
Article11. Boudreau AC, Wolf ME. 2005; Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 25:9144–9151. DOI: 10.1523/JNEUROSCI.2252-05.2005. PMID: 16207873. PMCID: PMC6725751.
Article12. Jedynak J, Hearing M, Ingebretson A, Ebner SR, Kelly M, Fischer RA, Kourrich S, Thomas MJ. 2016; Cocaine and amphetamine induce overlapping but distinct patterns of AMPAR plasticity in nucleus accumbens medium spiny neurons. Neuropsychopharmacology. 41:464–476. DOI: 10.1038/npp.2015.168. PMID: 26068728. PMCID: PMC5130122.
Article13. Harms KJ, Tovar KR, Craig AM. 2005; Synapse-specific regulation of AMPA receptor subunit composition by activity. J Neurosci. 25:6379–6388. DOI: 10.1523/JNEUROSCI.0302-05.2005. PMID: 16000628. PMCID: PMC6725282.
Article14. Melone M, Burette A, Weinberg RJ. 2005; Light microscopic identification and immunocytochemical characterization of glutamatergic synapses in brain sections. J Comp Neurol. 492:495–509. DOI: 10.1002/cne.20743. PMID: 16228991.
Article15. Notter T, Panzanelli P, Pfister S, Mircsof D, Fritschy JM. 2014; A protocol for concurrent high-quality immunohistochemical and biochemical analyses in adult mouse central nervous system. Eur J Neurosci. 39:165–175. DOI: 10.1111/ejn.12447. PMID: 24325300.
Article16. Schneider Gasser EM, Straub CJ, Panzanelli P, Weinmann O, Sassoè-Pognetto M, Fritschy JM. 2006; Immunofluorescence in brain sections: simultaneous detection of presynaptic and postsynaptic proteins in identified neurons. Nat Protoc. 1:1887–1897. DOI: 10.1038/nprot.2006.265. PMID: 17487173.
Article17. Fritschy JM, Weinmann O, Wenzel A, Benke D. 1998; Synapse-specific localization of NMDA and GABA(A) receptor subunits revealed by antigen-retrieval immunohistochemistry. J Comp Neurol. 390:194–210. DOI: 10.1002/(SICI)1096-9861(19980112)390:2<194::AID-CNE3>3.0.CO;2-X. PMID: 9453664.
Article18. Watanabe M, Fukaya M, Sakimura K, Manabe T, Mishina M, Inoue Y. 1998; Selective scarcity of NMDA receptor channel subunits in the stratum lucidum (mossy fibre-recipient layer) of the mouse hippocampal CA3 subfield. Eur J Neurosci. 10:478–487. DOI: 10.1046/j.1460-9568.1998.00063.x. PMID: 9749710.
Article19. Henley JM, Wilkinson KA. 2016; Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neurosci. 17:337–350. DOI: 10.1038/nrn.2016.37. PMID: 27080385.
Article20. Reimers JM, Milovanovic M, Wolf ME. 2011; Quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions. Brain Res. 1367:223–233. DOI: 10.1016/j.brainres.2010.10.016. PMID: 20946890. PMCID: PMC3005033.
Article21. Malinow R, Mainen ZF, Hayashi Y. 2000; LTP mechanisms: from silence to four-lane traffic. Curr Opin Neurobiol. 10:352–357. DOI: 10.1016/S0959-4388(00)00099-4. PMID: 10851179.
Article22. Santos SD, Carvalho AL, Caldeira MV, Duarte CB. 2009; Regulation of AMPA receptors and synaptic plasticity. Neuroscience. 158:105–125. DOI: 10.1016/j.neuroscience.2008.02.037. PMID: 18424006.
Article23. Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. 2008; CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 11:344–353. DOI: 10.1038/nn2054. PMID: 18278040.
Article24. Paxinos G, Watson C. 2004. The rat brain in stereotaxic coordinates. 5th ed. Elsevier Academic Press;London:25. Sheng M, Kim E. 2011; The postsynaptic organization of synapses. Cold Spring Harb Perspect Biol. 3:a005678. DOI: 10.1101/cshperspect.a005678. PMID: 22046028. PMCID: PMC3225953.
Article26. Yoon HS, Kim S, Park HK, Kim JH. 2007; Microinjection of CART peptide 55-102 into the nucleus accumbens blocks both the expression of behavioral sensitization and ERK phosphorylation by cocaine. Neuropharmacology. 53:344–351. DOI: 10.1016/j.neuropharm.2007.05.014. PMID: 17610912.
Article27. Kim WY, Jang JK, Lee JW, Jang H, Kim JH. 2013; Decrease of GSK3β phosphorylation in the rat nucleus accumbens core enhances cocaine-induced hyper-locomotor activity. J Neurochem. 125:642–648. DOI: 10.1111/jnc.12222. PMID: 23439225.
Article28. Ferrario CR, Li X, Wang X, Reimers JM, Uejima JL, Wolf ME. 2010; The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacology. 35:818–833. DOI: 10.1038/npp.2009.190. PMID: 19924109. PMCID: PMC3014646.
Article29. Malinow R, Malenka RC. 2002; AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 25:103–126. DOI: 10.1146/annurev.neuro.25.112701.142758. PMID: 12052905.
Article30. Triller A, Choquet D. 2005; Surface trafficking of receptors between synaptic and extrasynaptic membranes: and yet they do move! Trends Neurosci. 28:133–139. DOI: 10.1016/j.tins.2005.01.001. PMID: 15749166.
Article31. Sassoé-Pognetto M, Utvik JK, Camoletto P, Watanabe M, Stephenson FA, Bredt DS, Ottersen OP. 2003; Organization of postsynaptic density proteins and glutamate receptors in axodendritic and dendrodendritic synapses of the rat olfactory bulb. J Comp Neurol. 463:237–248. DOI: 10.1002/cne.10745. PMID: 12820158.
Article32. Wouterlood FG, Böckers T, Witter MP. 2003; Synaptic contacts between identified neurons visualized in the confocal laser scanning microscope. Neuroanatomical tracing combined with immunofluorescence detection of post-synaptic density proteins and target neuron-markers. J Neurosci Methods. 128:129–142. DOI: 10.1016/S0165-0270(03)00171-7. PMID: 12948556.33. Wolf ME. 2016; Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci. 17:351–365. DOI: 10.1038/nrn.2016.39. PMID: 27150400. PMCID: PMC5466704.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cocaine-Induced Behavioral Sensitization in Mice: Effects of Microinjection of Dopamine D2 Receptor Antagonist into the Nucleus Accumbens
- Sequential Activation of AMPA Receptors and Glial Cells in a Pain Model of Lumbar Spine Disc Herniation
- Blockade of ERK Phosphorylation in the Nucleus Accumbens Inhibits the Expression of Cocaine-induced Behavioral Sensitization in Rats
- The Role of AMPA Receptors in Synaptic Plasticity by Drugs of Abuse
- Effect of Ginseng Saponins on Nicotine-Induced Dopamine Release in the Rat Nucleus Accumbens and Striatum