Ann Clin Microbiol.
2020 Dec;23(4):233-239. 10.5145/ACM.2020.23.4.2.
Cross-Correlation Analysis of the Incidence of Multidrug-Resistant Organisms with Hand Hygiene Compliance and Effectiveness of Alcohol-Gel Hand Hygiene Practice
- Affiliations
-
- 1Infection Control Team, Gyeongsang National University Changwon Hospital, Changwon, Korea
- 2Department of Laboratory Medicine, Gyeongsang National University Changwon Hospital, Changwon, Korea
- 3Institute of Health Science Gyeongsang National University, Jinju, Korea
- KMID: 2509648
- DOI: http://doi.org/10.5145/ACM.2020.23.4.2
Abstract
- Background
Multidrug- resistant organisms (MDRO) are a serious concern in healthcareassociated infections. Hand hygiene (HH) is essential to prevent the spread of MDRO in the healthcare institutes. The aim of this study was to investigate the relationship between the incidence of MDRO and hand hygiene compliance and the experimental effectiveness of alcohol-gel hand hygiene practice.
Methods
From March 2016 to September 2018, we analyzed the cross-correlation between the incidence of MDRO and the HH compliance each month at Gyeongsang National University Changwon Hospital. We employed an experiment to observe the effect of alcohol gel hand hygiene practice on the reduction of organisms on the hand surface using the handagar plates.
Results
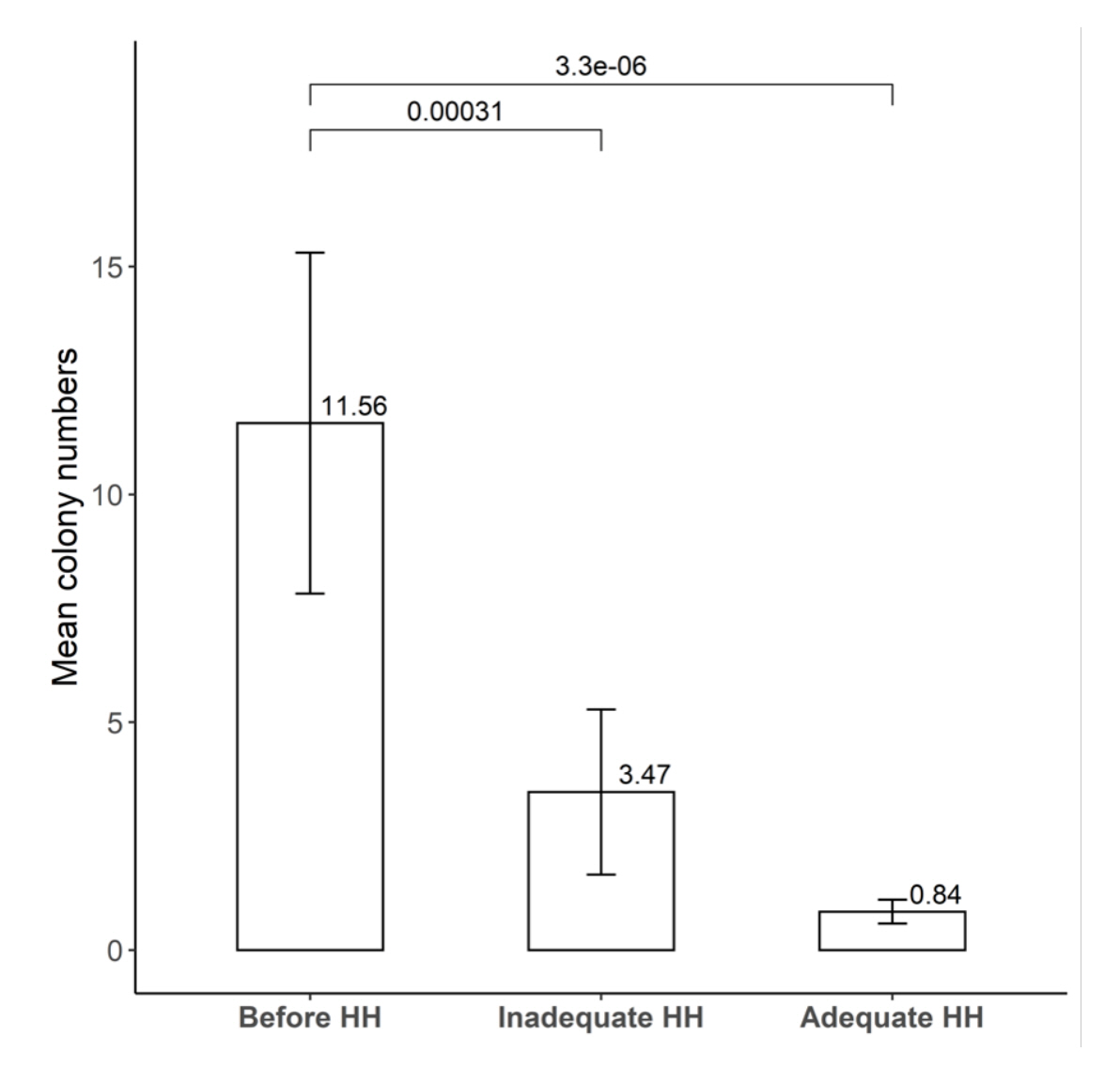
Among the MDRO, only vancomycin-resistant enterococci (VRE) showed a moderate correlation with the HH rate (r = 0.55). The hand-agar plate experiment showed a significant bacterial reduction for inadequate HH (mean 3.47 CFU) and optimal HH (mean, 0.84 CFU) than before HH (mean, 11.56 CFU) (n = 32, P = 0.006).
Conclusion
The incidence of VRE showed a moderate correlation with HH among MDRO in the longitudinal analysis. HH practice was more effective in preventing the spread of VRE compared with other MDRO in our institute. Optimal alcohol-gel HH practice can effectively remove bacteria on the hand surface.
Figure
Reference
-
1. Suarez C, Pena C, Arch O, Dominguez MA, Tubau F, Juan C, et al. A large sustained endemic outbreak of multiresistant Pseudomonas aeruginosa: a new epidemiological scenario for nosocomial acquisition. BMC Infect Dis 2011;11:272.2. Link T. Guideline implementation: transmission-based precautions. AORN J 2019;110:637-49.3. Pena C, Pujol M, Ardanuy C, Ricart A, Pallares R, Linares J, et al. Epidemiology and successful control of a large outbreak due to Klebsiella pneumoniae producing extendedspectrum beta-lactamases. Antimicrob Agents Chemother 1998;42:53-8.4. De Waele JJ, Boelens J, Leroux-Roels I. Multidrug-resistant bacteria in ICU: fact or myth. Curr Opin Anaesthesiol 2020;33:156-61.5. World Health Organization. WHO guidelines on hand hygiene in health care: first global patient safety challenge clean care is safer care. World Health Organization; 2009.6. Sickbert-Bennett EE, DiBiase LM, Willis TM, Wolak ES, Weber DJ, Rutala WA. Reduction of healthcare-associated infections by exceeding high compliance with hand hygiene practices. Emerg Infect Dis 2016;22:1628-30.7. Sax H, Allegranzi B, Uckay I, Larson E, Boyce J, Pittet D. 'My five moments for hand hygiene': a user-centred design approach to understand, train, monitor and report hand hygiene. J Hosp Infect 2007;67:9-21.8. Baek EH, Kim SE, Kim DH, Cho OH, Hong SI, Kim S. The difference in hand hygiene compliance rate between unit-based observers and trained observers for World Health Organization checklist and optimal hand hygiene. Int J Infect Dis 2020;90:197-200.9. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; 29th ed. CLSI supplement M100. Wayne; PA: 2019.10. Marimuthu K, Pittet D, Harbarth S. The effect of improved hand hygiene on nosocomial MRSA control. Antimicrob Resist Infect Control 2014;3:34.11. Apisarnthanarak A, Pinitchai U, Thongphubeth K, Yuekyen C, Warren DK, Fraser VJ, et al. A multifaceted intervention to reduce pandrug-resistant Acinetobacter baumannii colonization and infection in 3 intensive care units in a Thai tertiary care center: a 3-year study. Clin Infect Dis 2008;47:760-7.12. Loftus RW, Koff MD, Brown JR, Patel HM, Jensen JT, Reddy S, et al. The dynamics of Enterococcus transmission from bacterial reservoirs commonly encountered by anesthesia providers. Anesth Analg 2015;120:827-36.13. Vaillant L, Birgand G, Esposito-Farese M, Astagneau P, Pulcini C, Robert J, et al. Awareness among French healthcare workers of the transmission of multidrug resistant organisms: a large cross-sectional survey. Antimicrob Resist Infect Control 2019;8:173.14. Lingawi H, Maher Y, Afifi I. Impact of educational intervention for hand hygiene on dental students' knowledge, attitude, and bacterial contamination level on hands. J Contemp Dent Pract 2017;18:1164-72.15. Jabeen K, Mal PB, Tharwani A, Hashmi M, Farooqi J. Persistence of Candida auris on latex and nitrile gloves with transmission to sterile urinary cathetersdouble dagger. Med Mycol 2020;58:128-32.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of a Scenario based Hand Hygiene Education Program on Hand Hygiene Knowledge, Hand Hygiene Perception, Hand Hygiene Compliance and Hand Hygiene Method in Nursing Students
- Comparison of Antimicrobial Effect of Alcohol Gel according to the Amount and Drying Time in Health Personnel Hand Hygiene
- Comparison of the Degree of Bacterial Removal by Hand Hygiene Products
- Hand Hygiene Promotion in a Hospital Setting through the WHO Multimodal Hand Hygiene Improvement Strategy
- A Three-Year Study of the Effectiveness of Hand-Hygiene Protocol Implementation at a University Hospital