J Korean Med Sci.
2020 Dec;35(47):e399. 10.3346/jkms.2020.35.e399.
Identification of Sleep Apnea Severity Based on Deep Learning from a Short-term Normal ECG
- Affiliations
-
- 1Institute of AI and Big Data in Medicine, Wonju College of Medicine, Yonsei University, Wonju, Korea
- 2Department of Biomedical Engineering, College of Health Science, Yonsei University, Wonju, Korea
- 3Department of Neurology, Samsung Medical Center, School of Medicine, Sungkyunkwan University, Seoul, Korea
- KMID: 2509443
- DOI: http://doi.org/10.3346/jkms.2020.35.e399
Abstract
- Background
This paper proposes a novel method for automatically identifying sleep apnea (SA) severity based on deep learning from a short-term normal electrocardiography (ECG) signal.
Methods
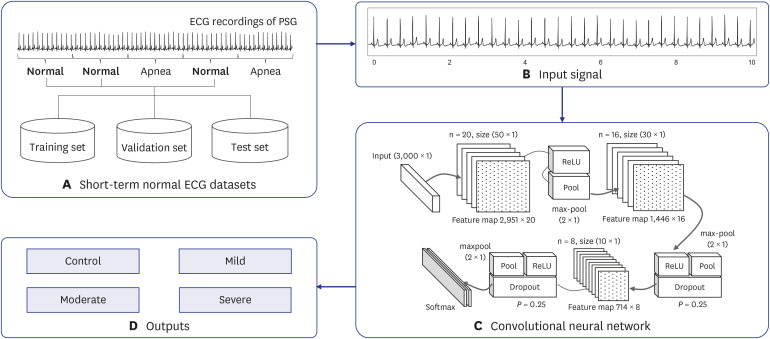
A convolutional neural network (CNN) was used as an identification model and implemented using a one-dimensional convolutional, pooling, and fully connected layer. An optimal architecture is incorporated into the CNN model for the precise identification of SA severity. A total of 144 subjects were studied. The nocturnal single-lead ECG signal was collected, and the short-term normal ECG was extracted from them. The short-term normal ECG was segmented for a duration of 30 seconds and divided into two datasets for training and evaluation. The training set consists of 82,952 segments (66,360 training set, 16,592 validation set) from 117 subjects, while the test set has 20,738 segments from 27 subjects.
Results
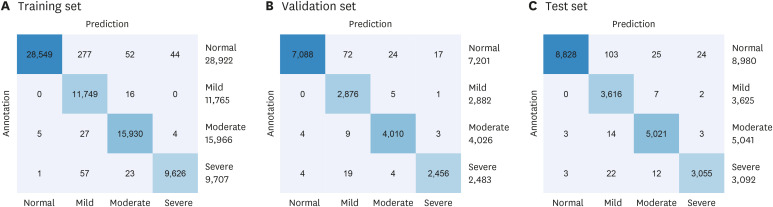
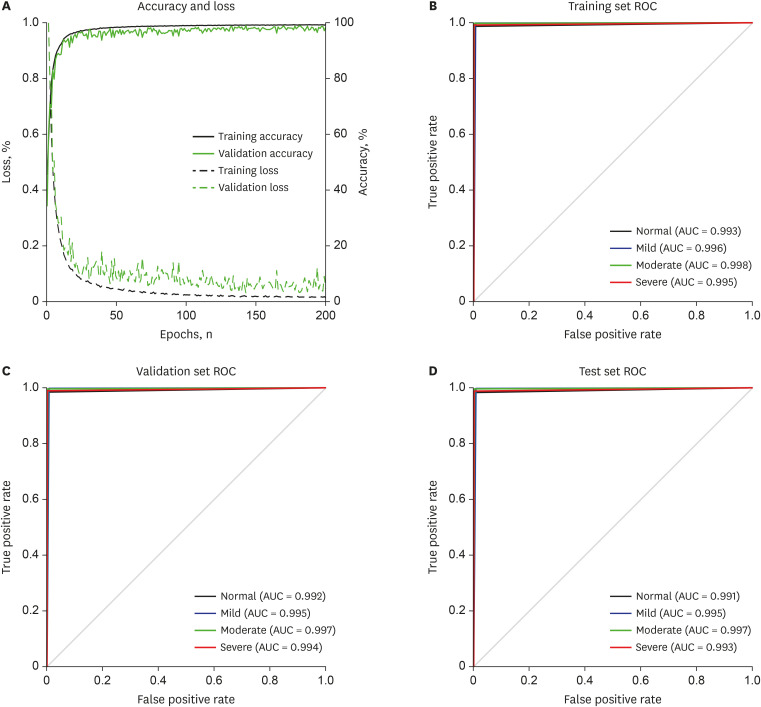
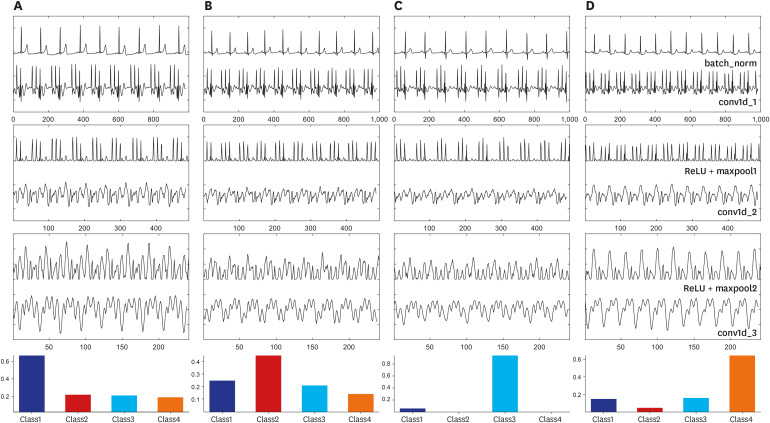
F1-score of 98.0% was obtained from the test set. Mild and moderate SA can be identified with an accuracy of 99.0%.
Conclusion
The results showed the possibility of automatically identifying SA severity based on a short-term normal ECG signal.
Keyword
Figure
Cited by 1 articles
-
A Retrospective Clinical Evaluation of an Artificial Intelligence Screening Method for Early Detection of STEMI in the Emergency Department
Dongsung Kim, Ji Eun Hwang, Youngjin Cho, Hyoung-Won Cho, Wonjae Lee, Ji Hyun Lee, Il-Young Oh, Sumin Baek, Eunkyoung Lee, Joonghee Kim
J Korean Med Sci. 2022;37(10):e81. doi: 10.3346/jkms.2022.37.e81.
Reference
-
1. Thorpy M. International classification of sleep disorders. In : Chokroverty S, editor. Sleep Disorders Medicine: Basic Science, Technical Considerations and Clinical Aspects. 4th ed. New York, NY: Springer;2017. p. 475–484.2. Šušmáková K. Human sleep and sleep EEG. Meas Sci Rev. 2004; 4(2):59–74.3. Banno K, Kryger MH. Sleep apnea: clinical investigations in humans. Sleep Med. 2007; 8(4):400–426. PMID: 17478121.
Article4. Chervin RD. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest. 2000; 118(2):372–379. PMID: 10936127.
Article5. Graff-Radford SB, Newman A. Obstructive sleep apnea and cluster headache. Headache. 2004; 44(6):607–610. PMID: 15186306.6. Lattimore JD, Celermajer DS, Wilcox I. Obstructive sleep apnea and cardiovascular disease. J Am Coll Cardiol. 2003; 41(9):1429–1437. PMID: 12742277.
Article7. Lal C, Strange C, Bachman D. Neurocognitive impairment in obstructive sleep apnea. Chest. 2012; 141(6):1601–1610. PMID: 22670023.
Article8. Freire AX, Kadaria D, Avecillas JF, Murillo LC, Yataco JC. Obstructive sleep apnea and immunity: relationship of lymphocyte count and apnea hypopnea index. South Med J. 2010; 103(8):771–774. PMID: 20622723.
Article9. Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002; 6(2):49–54. PMID: 12075479.
Article10. Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest. 2007; 132(1):325–337. PMID: 17625094.11. Douglas NJ, Thomas S, Jan MA. Clinical value of polysomnography. Lancet. 1992; 339(8789):347–350. PMID: 1346422.
Article12. de Chazal P, Penzel T, Heneghan C. Automated detection of obstructive sleep apnoea at different time scales using the electrocardiogram. Physiol Meas. 2004; 25(4):967–983. PMID: 15382835.13. Bacharova L, Triantafyllou E, Vazaios C, Tomeckova I, Paranicova I, Tkacova R. The effect of obstructive sleep apnea on QRS complex morphology. J Electrocardiol. 2015; 48(2):164–170. PMID: 25541278.
Article14. Mendez MO, Bianchi AM, Matteucci M, Cerutti S, Penzel T. Sleep apnea screening by autoregressive models from a single ECG lead. IEEE Trans Biomed Eng. 2009; 56(12):2838–2850. PMID: 19709961.
Article15. Mendez MO, Corthout J, Van Huffel S, Matteucci M, Penzel T, Cerutti S, et al. Automatic screening of obstructive sleep apnea from the ECG based on empirical mode decomposition and wavelet analysis. Physiol Meas. 2010; 31(3):273–289. PMID: 20086277.
Article16. Chen L, Zhang X, Song C. An automatic screening approach for obstructive sleep apnea diagnosis based on single-lead electrocardiogram. IEEE Trans Autom Sci Eng. 2015; 12(1):106–115.
Article17. Miotto R, Wang F, Wang S, Jiang X, Dudley JT. Deep learning for healthcare: review, opportunities and challenges. Brief Bioinform. 2018; 19(6):1236–1246. PMID: 28481991.
Article18. He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. In : Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition; 2016 June 27-30; Las Vegas, NV. Piscataway, NJ: Institute of Electrical and Electronics Engineers;2016. p. 770–778. DOI: 10.1109/CVPR.2016.90.19. Wu R, Yan S, Shan Y, Dang Q, Sun G. . Deep image: scaling up image recognition. arXiv. 2015; 1501.02876.20. Kiranyaz S, Ince T, Gabbouj M. Real-time patient-specific ECG classification by 1-D convolutional neural networks. IEEE Trans Biomed Eng. 2016; 63(3):664–675. PMID: 26285054.
Article21. Dey D, Chaudhuri S, Munshi S. Obstructive sleep apnoea detection using convolutional neural network based deep learning framework. Biomed Eng Lett. 2017; 8(1):95–100. PMID: 30603194.
Article22. Urtnasan E, Park JU, Lee KJ. Multiclass classification of obstructive sleep apnea/hypopnea based on a convolutional neural network from a single-lead electrocardiogram. Physiol Meas. 2018; 39(6):065003. PMID: 29794342.
Article23. Berry RB, Quan SF, Abreu A. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, IL: American Academy of Sleep Medicine;2012.24. Schmidhuber J. Deep learning in neural networks: an overview. Neural Netw. 2015; 61:85–117. PMID: 25462637.
Article25. van Laarhoven T. L2 regularization versus batch and weight normalization. arXiv. 2017; 1706.05350.26. Srivastava N, Hinton G, Krizhevsky A, Sutskever I, Salakhutdinov R. Dropout: a simple way to prevent neural networks from overfitting. J Mach Learn Res. 2014; 15(56):1929–1958.27. Zeiler MD, Ranzato M, Monga R, Mao M, Yang K, Le QV, et al. On rectified linear units for speech processing. In : Proceedings of the 2013 IEEE International Conference on Acoustics, Speech and Signal Processing; 2013 May 26-31; Vancouver, Canada. Piscataway, NJ: Institute of Electrical and Electronics Engineers;2013. p. 3517–3521. DOI: 10.1109/ICASSP.2013.6638312.28. Zou F, Shen L, Jie Z, Sun J, Liu W. Weighted AdaGrad with unified momentum. arXiv. 2018; 1808.03408.29. Keras: the Python deep learning API. Updated 2015. Accessed March 24, 2019. https://keras.io/.30. Abadi M, Agarwal A, Barham P, Brevdo E, Chen Z, Citro C, et al. TensorFlow: large-scale machine learning on heterogeneous distributed systems. arXiv. 2016; 1603.04467.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Medical Treatment of Sleep Apnea Syndrome
- The influence of sleep and sleep apnea in memory function
- Automatic Prediction of Atrial Fibrillation Based on Convolutional Neural Network Using a Short-term Normal Electrocardiogram Signal
- Identification of Atrial Fibrillation With Single-Lead Mobile ECG During Normal Sinus Rhythm Using Deep Learning
- Long-Term Side Effects of Mandibular Advancement Devices in Patients with Obstructive Sleep Apnea