Int J Stem Cells.
2020 Nov;13(3):353-363. 10.15283/ijsc20061.
Screening of Integrin Heterodimers Expressed Functionally on the Undifferentiated Spermatogonial Stem Cells in the Outbred ICR Mice
- Affiliations
-
- 1Department of Animal Life Science, Kangwon National University, Chuncheon, Korea
- 2KustoGen Inc., Chuncheon, Korea
- 3Department of Animal Science, Chonnam National University, Gwangju, Korea
- 4Optipharm Inc., Cheongju, Korea
- 5College of Veterinary Medicine, Kangwon National University, Chuncheon, Korea
- 6Department of Applied Animal Science, Kangwon National University, Chuncheon, Korea
- KMID: 2508909
- DOI: http://doi.org/10.15283/ijsc20061
Abstract
- Background and Objectives
Outbred mice are widely used in toxicology, pharmacology, and fundamental biomedical research. However, there have been no reports of in vitro culture systems for spermatogonial stem cells (SSCs) derived from these mice.
Methods
As a step towards constructing a non-cellular niche supporting the in vitro maintenance of outbred mouse SSC self-renewal, we systematically investigated the types of integrin heterodimers that are expressed transcriptionally, translationally, and functionally in SSCs derived from Imprinting Control Region (ICR) mice.
Results
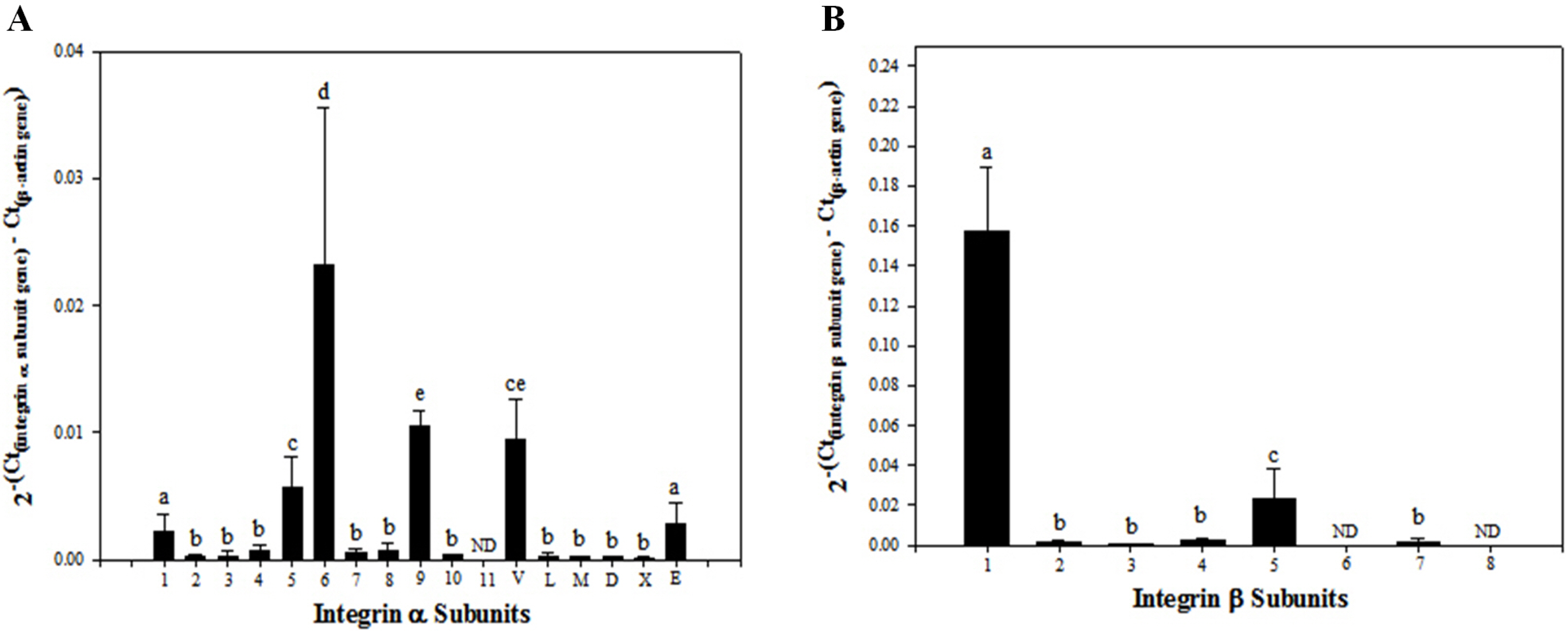
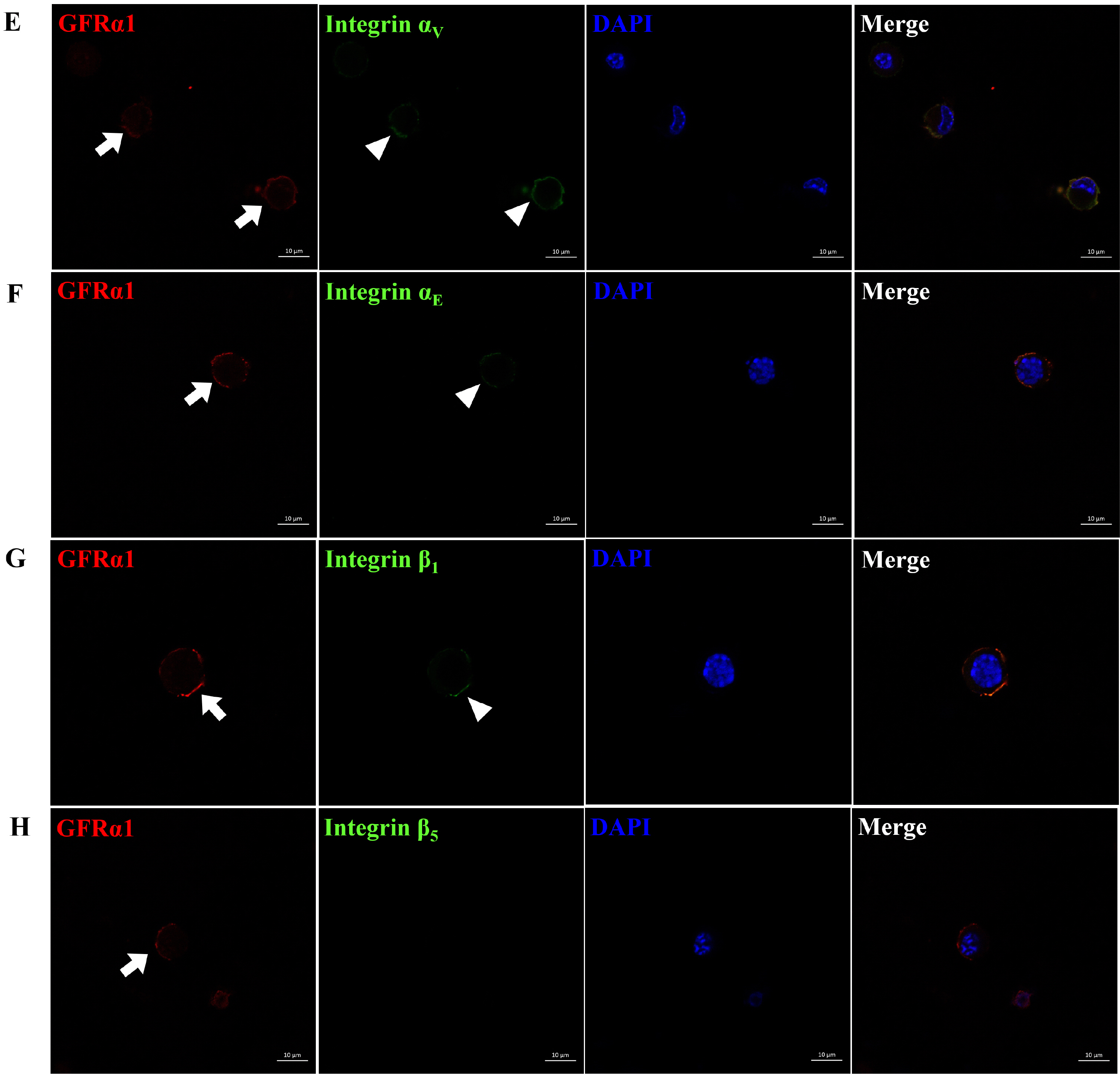
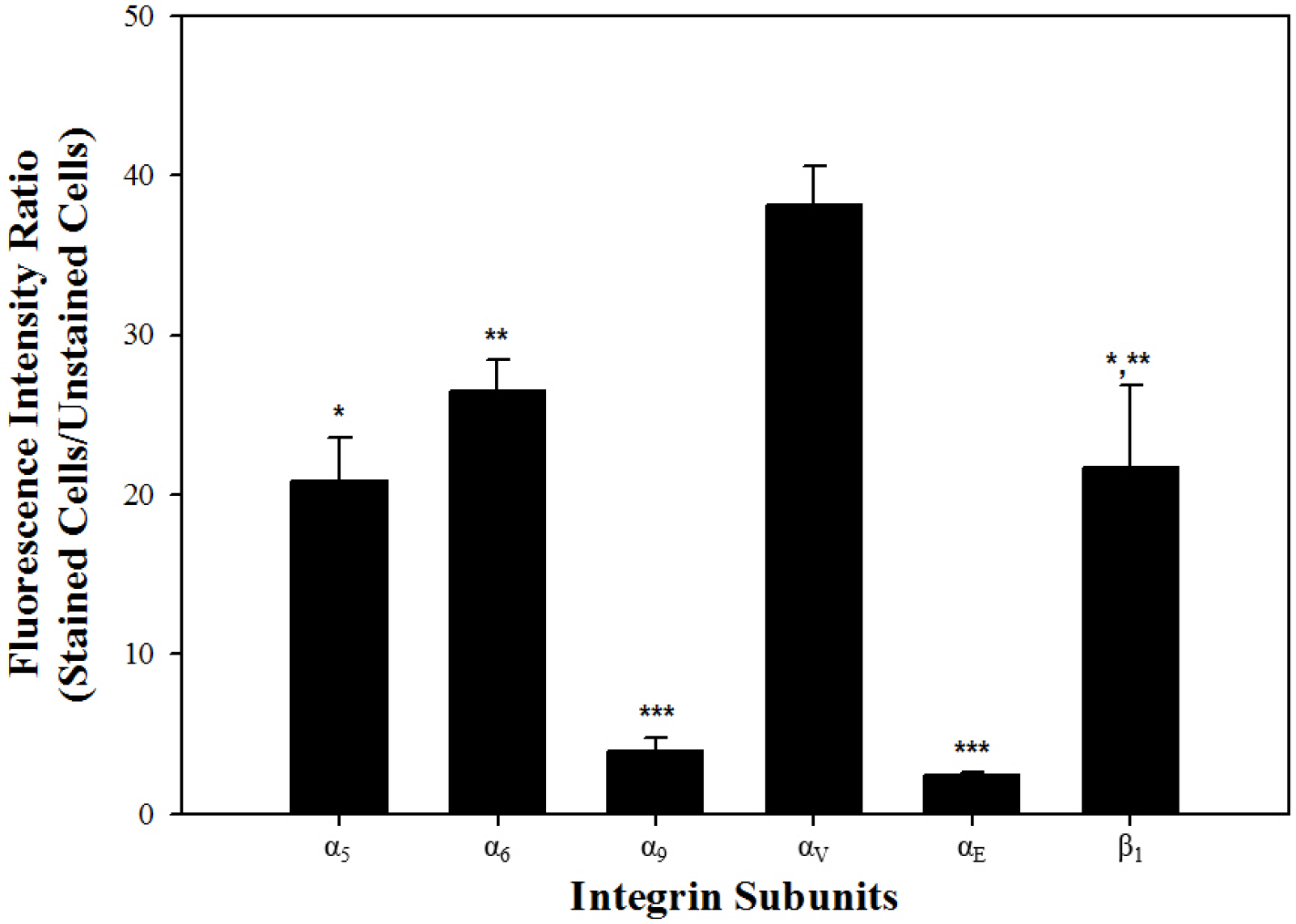
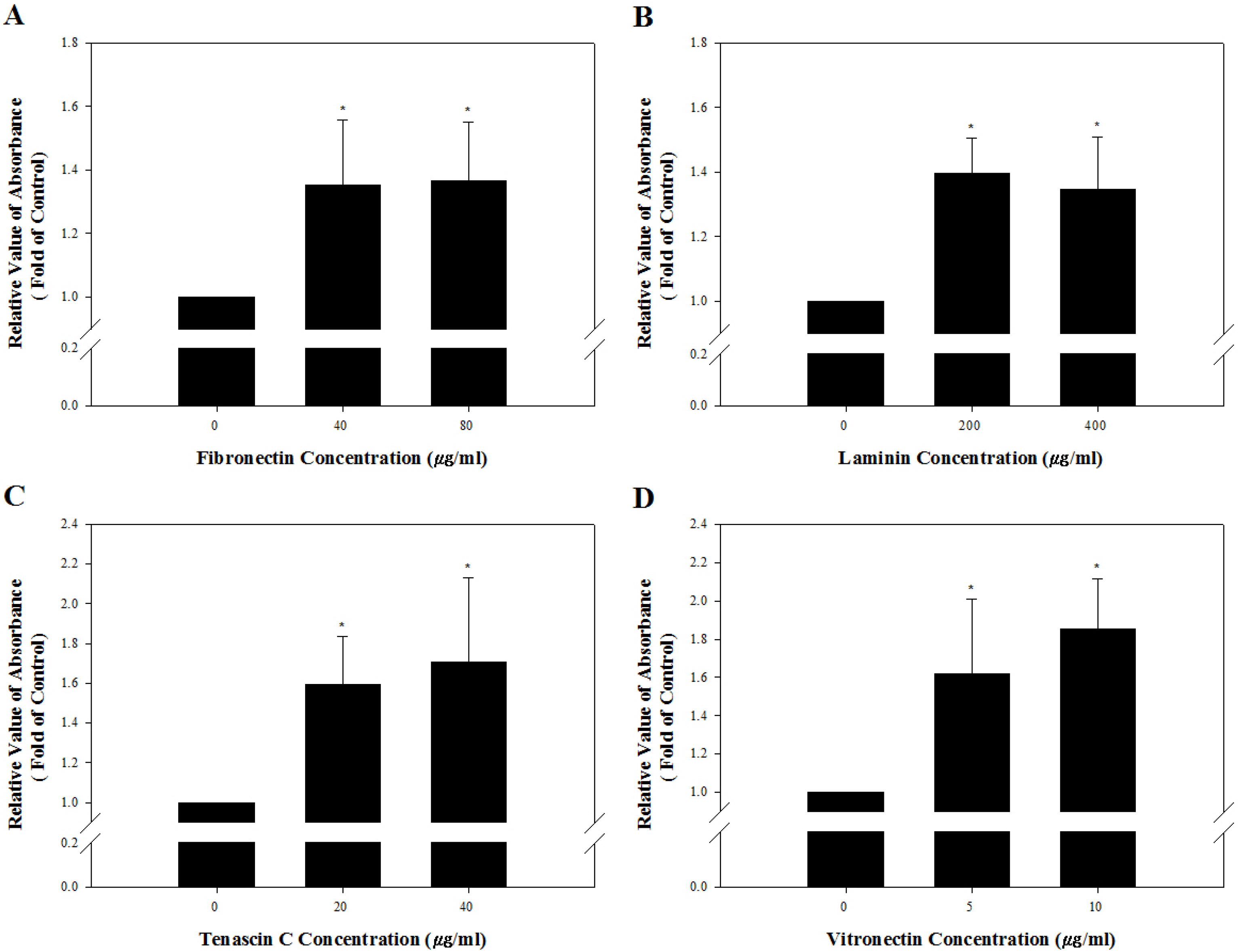
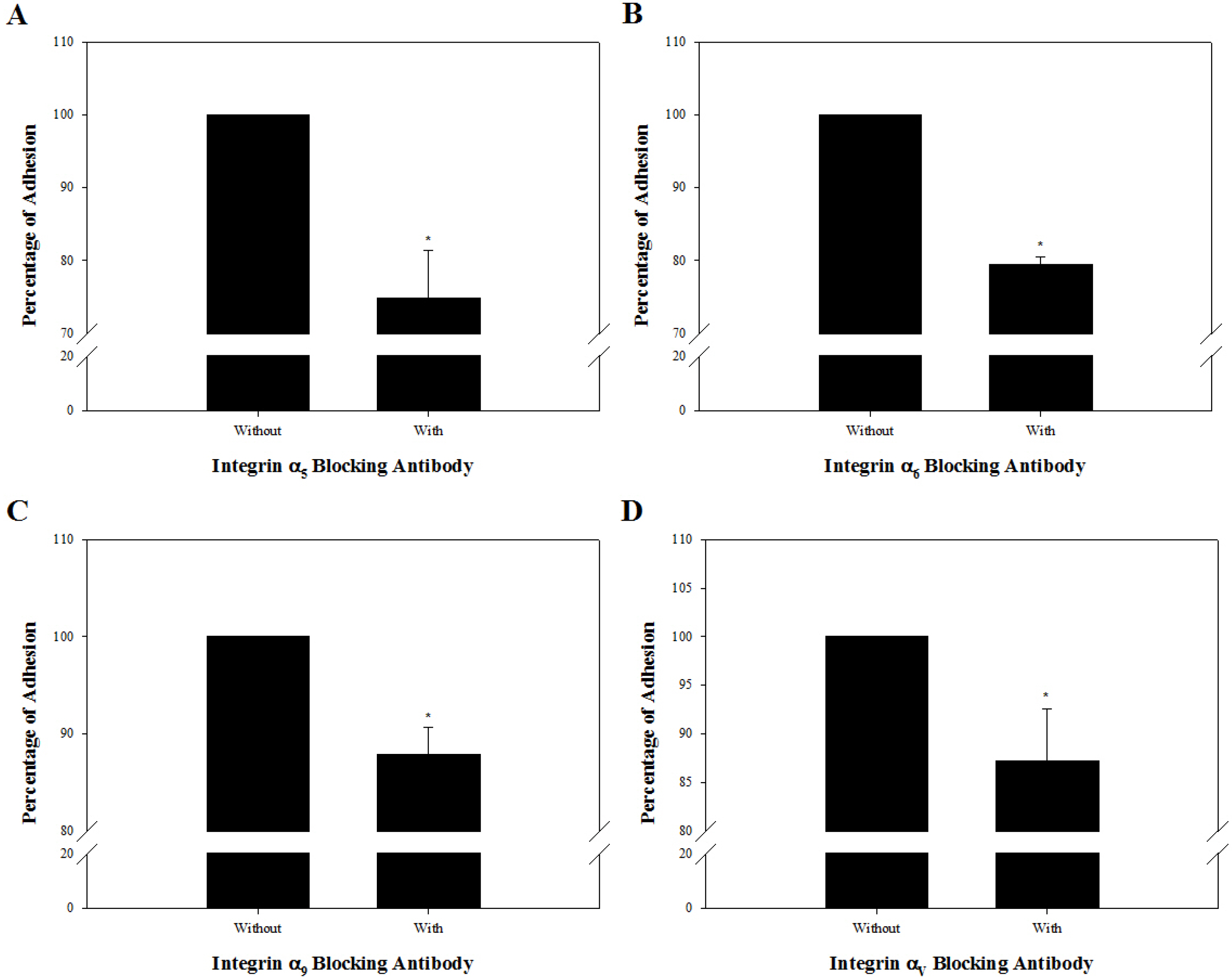
Among the genes encoding 25 integrin subunits, integrin α1, α5, α6, α9, αV, and αE, and integrin β1 and β5 had significantly higher transcriptional levels than the other subunits. Furthermore, at the translational level, integrin α5, α6, α9, αV, and αE, and β1 were localized on the surface of SSCs, but integrin α1 and β5 not. Moreover, significantly stronger translational expression than integrin α9 and αE was observed in integrin α5, α6, αV, and β1. SSCs showed significantly increased adhesion to fibronectin, laminin, tenascin C and vitronectin, and functional blocking of integrin α5β1, α6β1, α9β1 or αVβ1 significantly inhibited adhesion to these molecules.
Conclusions
We confirmed that integrin α5β1, α6β1, α9β1 and αVβ1 actively function on the surface of undifferentiated SSCs derived from outbred ICR mice.
Keyword
Figure
Reference
-
References
1. Liu N, Lu M, Tian X, Han Z. 2007; Molecular mechanisms involved in self-renewal and pluripotency of embryonic stem cells. J Cell Physiol. 211:279–286. DOI: 10.1002/jcp.20978. PMID: 17195167.
Article2. Saunders A, Faiola F, Wang J. 2013; Concise review: pursuing self-renewal and pluripotency with the stem cell factor Nanog. Stem Cells. 31:1227–1236. DOI: 10.1002/stem.1384. PMID: 23653415. PMCID: PMC3706551.
Article3. Chen S, Lewallen M, Xie T. 2013; Adhesion in the stem cell niche: biological roles and regulation. Development. 140:255–265. DOI: 10.1242/dev.083139. PMID: 23250203. PMCID: PMC3597204.
Article4. Lane SW, Williams DA, Watt FM. 2014; Modulating the stem cell niche for tissue regeneration. Nat Biotechnol. 32:795–803. DOI: 10.1038/nbt.2978. PMID: 25093887. PMCID: PMC4422171.
Article5. Gattazzo F, Urciuolo A, Bonaldo P. 2014; Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 1840:2506–2519. DOI: 10.1016/j.bbagen.2014.01.010. PMID: 24418517. PMCID: PMC4081568.
Article6. Rompolas P, Mesa KR, Greco V. 2013; Spatial organization within a niche as a determinant of stem-cell fate. Nature. 502:513–518. DOI: 10.1038/nature12602. PMID: 24097351. PMCID: PMC3895444.
Article7. Ahn S, Lee KY, Parker KK, Shin K. 2018; Formation of multi-component extracellular matrix protein fibers. Sci Rep. 8:1913. DOI: 10.1038/s41598-018-20371-8. PMID: 29382927. PMCID: PMC5790006.
Article8. Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. 2016; Extracellular matrix structure. Adv Drug Deliv Rev. 97:4–27. DOI: 10.1016/j.addr.2015.11.001. PMID: 26562801.
Article9. Schaefer L, Reinhardt DP. 2016; Special issue: Extracellular matrix: therapeutic tools and targets in cancer treatment. Adv Drug Deliv Rev. 97:1–3. DOI: 10.1016/j.addr.2016.01.001. PMID: 26872878.
Article10. Bökel C, Brown NH. 2002; Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev Cell. 3:311–321. DOI: 10.1016/S1534-5807(02)00265-4. PMID: 12361595.11. Seguin L, Desgrosellier JS, Weis SM, Cheresh DA. 2015; Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 25:234–240. DOI: 10.1016/j.tcb.2014.12.006. PMID: 25572304. PMCID: PMC4380531.
Article12. Vecino E, Heller JP, Veiga-Crespo P, Martin KR, Fawcett JW. 2015; Influence of extracellular matrix components on the expression of integrins and regeneration of adult retinal ganglion cells. PLoS One. 10:e0125250. DOI: 10.1371/journal.pone.0125250. PMID: 26018803. PMCID: PMC4446304.
Article13. Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, Hasenfuss G. 2006; Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 440:1199–1203. DOI: 10.1038/nature04697. PMID: 16565704.
Article14. Lovasco LA, Gustafson EA, Seymour KA, de Rooij DG, Freiman RN. 2015; TAF4b is required for mouse spermatogonial stem cell development. Stem Cells. 33:1267–1276. DOI: 10.1002/stem.1914. PMID: 25727968. PMCID: PMC4376611.
Article15. Takashima S, Kanatsu-Shinohara M, Tanaka T, Morimoto H, Inoue K, Ogonuki N, Jijiwa M, Takahashi M, Ogura A, Shinohara T. 2015; Functional differences between GDNF-dependent and FGF2-dependent mouse spermatogonial stem cell self-renewal. Stem Cell Reports. 4:489–502. DOI: 10.1016/j.stemcr.2015.01.010. PMID: 25684228. PMCID: PMC4375941.
Article16. Han NR, Park YH, Yun JI, Park HJ, Park MH, Kim MS, Choi JH, Lee E, Gong SP, Lim JM, Lee ST. 2014; Determination of feeder cell-based cellular niches supporting the colonization and maintenance of spermatogonial stem cells from prepubertal domestic cat testes. Reprod Domest Anim. 49:705–710. DOI: 10.1111/rda.12351. PMID: 24978324.
Article17. Lee KH, Lee WY, Kim JH, Park CK, Do JT, Kim JH, Choi YS, Kim NH, Song H. 2016; Subculture of germ cell-derived colonies with GATA4-positive feeder cells from neonatal pig testes. Stem Cells Int. 2016:6029271. DOI: 10.1155/2016/6029271. PMID: 26880974. PMCID: PMC4736562.
Article18. Park MH, Park JE, Kim MS, Lee KY, Yun JI, Choi JH, Lee E, Lee ST. 2014; Identification of niche conditions supporting short-term culture of spermatogonial stem cells derived from porcine neonatal testis. J Emb Trans. 29:221–228. DOI: 10.12750/JET.2014.29.3.221.
Article19. He BR, Lu F, Zhang L, Hao DJ, Yang H. 2015; An alternative long-term culture system for highly-pure mouse spermatogonial stem cells. J Cell Physiol. 230:1365–1375. DOI: 10.1002/jcp.24880. PMID: 25503338.
Article20. Yalcin B, Nicod J, Bhomra A, Davidson S, Cleak J, Farinelli L, Østerås M, Whitley A, Yuan W, Gan X, Goodson M, Klenerman P, Satpathy A, Mathis D, Benoist C, Adams DJ, Mott R, Flint J. 2010; Commercially available outbred mice for genome-wide association studies. PLoS Genet. 6:e1001085. DOI: 10.1371/journal.pgen.1001085. PMID: 20838427. PMCID: PMC2932682.
Article21. Aldinger KA, Sokoloff G, Rosenberg DM, Palmer AA, Millen KJ. 2009; Genetic variation and population substructure in outbred CD-1 mice: implications for genome-wide association studies. PLoS One. 4:e4729. DOI: 10.1371/journal.pone.0004729. PMID: 19266100. PMCID: PMC2649211.
Article22. Gatti DM, Svenson KL, Shabalin A, Wu LY, Valdar W, Simecek P, Goodwin N, Cheng R, Pomp D, Palmer A, Chesler EJ, Broman KW, Churchill GA. 2014; Quantitative trait locus mapping methods for diversity outbred mice. G3 (Bethesda). 4:1623–1633. DOI: 10.1534/g3.114.013748. PMID: 25237114. PMCID: PMC4169154.
Article23. Chia R, Achilli F, Festing MF, Fisher EM. 2005; The origins and uses of mouse outbred stocks. Nat Genet. 37:1181–1186. DOI: 10.1038/ng1665. PMID: 16254564.
Article24. Hynes RO. 1992; Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 69:11–25. DOI: 10.1016/0092-8674(92)90115-S. PMID: 1555235.
Article25. Meistrich ML, Shetty G. 2015; The new director of "the spermatogonial niche": introducing the peritubular macrophage. Cell Rep. 12:1069–1070. DOI: 10.1016/j.celrep.2015.07.057. PMID: 26287751.
Article26. Schwartz MA. 2010; Integrins and extracellular matrix in mecha-notransduction. Cold Spring Harb Perspect Biol. 2:a005066. DOI: 10.1101/cshperspect.a005066. PMID: 21084386. PMCID: PMC2982167.
Article27. Baert Y, Stukenborg JB, Landreh M, De Kock J, Jörnvall H, Söder O, Goossens E. 2015; Derivation and characterization of a cytocompatible scaffold from human testis. Hum Reprod. 30:256–267. DOI: 10.1093/humrep/deu330. PMID: 25505010.
Article28. Yan HH, Cheng CY. 2006; Laminin alpha 3 forms a complex with beta3 and gamma3 chains that serves as the ligand for alpha 6beta1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem. 281:17286–17303. DOI: 10.1074/jbc.M513218200. PMID: 16608848.
Article29. Glattauer V, Irving-Rodgers HF, Rodgers RJ, Stockwell S, Brownlee AG, Werkmeister JA, Ramshaw JA. 2007; Examination of basement membrane components associated with the bovine seminiferous tubule basal lamina. Reprod Fertil Dev. 19:473–481. DOI: 10.1071/RD06013. PMID: 17394796.
Article30. Harvey SJ, Perry J, Zheng K, Chen D, Sado Y, Jefferson B, Ninomiya Y, Jacobs R, Hudson BG, Thorner PS. 2006; Sequential expression of type IV collagen networks: testis as a model and relevance to spermatogenesis. Am J Pathol. 168:1587–1597. DOI: 10.2353/ajpath.2006.050816. PMID: 16651625. PMCID: PMC1606577.
Article31. Gersdorff N, Kohfeldt E, Sasaki T, Timpl R, Miosge N. 2005; Laminin gamma3 chain binds to nidogen and is located in murine basement membranes. J Biol Chem. 280:22146–22153. DOI: 10.1074/jbc.M501875200. PMID: 15824114.
Article32. Siu MK, Cheng CY. 2008; Extracellular matrix and its role in spermatogenesis. Adv Exp Med Biol. 636:74–91. DOI: 10.1007/978-0-387-09597-4_5. PMID: 19856163. PMCID: PMC4035910.
Article33. Miqueloto CA, Zorn TM. 2007; Characterization and distribution of hyaluronan and the proteoglycans decorin, biglycan and perlecan in the developing embryonic mouse gonad. J Anat. 211:16–25. DOI: 10.1111/j.1469-7580.2007.00741.x. PMID: 17543016. PMCID: PMC2375803.
Article34. Nuovo GJ, Preissner KT, Bronson RA. 1995; PCR-amplified vitronectin mRNA localizes in situ to spermatocytes and round spermatids in the human testis. Hum Reprod. 10:2187–2191. DOI: 10.1093/oxfordjournals.humrep.a136266. PMID: 8567871.
Article35. Giebel J, Löster K, Rune GM. 1997; Localization of integrin beta 1, alpha 1, alpha 5 and alpha 9 subunits in the rat testis. Int J Androl. 20:3–9. DOI: 10.1046/j.1365-2605.1997.d01-105.x. PMID: 9202984.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of commonly used ICR stocks and the characterization of Korl:ICR
- Establishment and Characterization of Multipotent Germ Line Stem Cells (MGSCs) from Neonatal Mouse Testis
- Strain Differences in the Chronic Mild Stress Animal Model of Depression and Anxiety in Mice
- A Study of Micronucleus Induction with Methyl Formate and 2-Methylbutane in Bone Marrow Cells of Male ICR Mice
- Inhibition of Class I Histone Deacetylase Enhances Self-Reprogramming of Spermatogonial Stem Cells into Pluripotent Stem Cells