Apprehensions about Excessive Belief in Digital Therapeutics: Points of Concern Excluding Merits
- Affiliations
-
- 1Department of Medical Informatics, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Endocrinology and Metabolism, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2508802
- DOI: http://doi.org/10.3346/jkms.2020.35.e373
Abstract
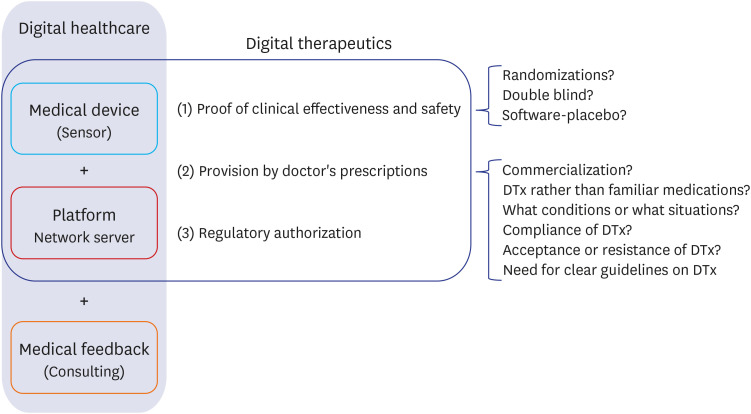
- Digital therapeutics (DTx), like drugs or medical devices, 1) must prove their effectiveness and safety through clinical trials; 2) are provided to patients through prescriptions from doctors; and 3) may require the approval of regulatory agencies, though this might not be mandatory. Although DTx will play an important role in the medical field in the near future, some merits of DTx have been exaggerated at this crucial juncture. In the medical field, where safety and effectiveness are important, merely reducing the development time and costs of DTx is not advantageous. The adverse effects of DTx are not yet well-known, and will be identified eventually, with the passage of time. DTx is beneficial for the collection and analysis of real-world data (RWD); however, they require new and distinct work to collect and analyze high-quality RWD. Naturally, whether this is possible must be independently ascertained through scientific methods. Depending on the type of disease, it is not recommended that DTx be prescribed, even if the patient rejects conventional treatment. Prescription of conventional pharmacotherapy is often necessary, and if the prescription of DTx is inadequate, the critical time for initial treatment may be missed. There is no basis for continuing DTx use by patients. Rather, the rate of continuity of DTx use is extremely low. While many conventional pharmacotherapies have undergone numerous verification and safety tests over a long time, barriers to the application of DTx in the medical field are lower than those for conventional pharmacotherapies. Considering these reasons, except for certain special cases, an approach to DTx is needed that complements the prescription of conventional pharmacotherapy by the medical staff. When DTx are prescribed by doctors who clearly know their advantages and disadvantages, the doctors' expertise may undergo further refinement, and the quality of medical care is expected to improve.
Keyword
Figure
Cited by 4 articles
-
Towards Telemedicine Adoption in Korea: 10 Practical Recommendations for Physicians
Hun-Sung Kim
J Korean Med Sci. 2021;36(17):e103. doi: 10.3346/jkms.2021.36.e103.Lack of Acceptance of Digital Healthcare in the Medical Market: Addressing Old Problems Raised by Various Clinical Professionals and Developing Possible Solutions
Jong Il Park, Hwa Young Lee, Hyunah Kim, Jisan Lee, Jiwon Shinn, Hun-Sung Kim
J Korean Med Sci. 2021;36(37):e253. doi: 10.3346/jkms.2021.36.e253.Reimbursement of Digital Therapeutics: Future Perspectives in Korea
Jin Han Ju, Boram Sim, Jeongeun Lee, Jin Yong Lee
Korean Circ J. 2022;52(4):265-279. doi: 10.4070/kcj.2022.0014.Expectations and concerns regarding medical advertisements via large commercial medical platform advertising companies: a legal perspective
Raeun Kim, Hakyoung Park, Jiwon Shinn, Hun-Sung Kim
Cardiovasc Prev Pharmacother. 2024;6(2):48-56. doi: 10.36011/cpp.2024.6.e8.
Reference
-
1. Sverdlov O, van Dam J, Hannesdottir K, Thornton-Wells T. Digital therapeutics: an integral component of digital innovation in drug development. Clin Pharmacol Ther. 2018; 104(1):72–80. PMID: 29377057.
Article2. Palanica A, Docktor MJ, Lieberman M, Fossat Y. The need for artificial intelligence in digital therapeutics. Digit Biomark. 2020; 4(1):21–25. PMID: 32399513.
Article3. Nordyke RJ, Appelbaum K, Berman MA. Estimating the impact of novel digital therapeutics in type 2 diabetes and hypertension: health economic analysis. J Med Internet Res. 2019; 21(10):e15814. PMID: 31599740.
Article4. Dorsey ER, Okun MS, Bloem BR. Care, convenience, comfort, confidentiality, and contagion: the 5 C's that will shape the future of telemedicine. J Parkinsons Dis. 2020; 10(3):893–897. PMID: 32538870.
Article5. Cho CH, Lee HJ. Could digital therapeutics be a game changer in psychiatry? Psychiatry Investig. 2019; 16(2):97–98.
Article6. Hirschtritt ME, Insel TR. Digital technologies in psychiatry: present and future. Focus Am Psychiatr Publ. 2018; 16(3):251–258. PMID: 31975919.
Article7. Choi MJ, Kim H, Nah HW, Kang DW. Digital therapeutics: emerging new therapy for neurologic deficits after stroke. J Stroke. 2019; 21(3):242–258. PMID: 31587534.
Article8. Kaufman N. Digital therapeutics: leading the way to improved outcomes for people with diabetes. Diabetes Spectr. 2019; 32(4):301–303. PMID: 31798286.
Article9. Afra P, Bruggers CS, Sweney M, Fagatele L, Alavi F, Greenwald M, et al. Mobile software as a medical device (SaMD) for the treatment of epilepsy: development of digital therapeutics comprising behavioral and music-based interventions for neurological disorders. Front Hum Neurosci. 2018; 12:171. PMID: 29780310.
Article10. Lougheed T. How “digital therapeutics” differ from traditional health and wellness apps. CMAJ. 2019; 191(43):E1200–1. PMID: 31659065.
Article11. Sharma A, Harrington RA, McClellan MB, Turakhia MP, Eapen ZJ, Steinhubl S, et al. Using digital health technology to better generate evidence and deliver evidence-based care. J Am Coll Cardiol. 2018; 71(23):2680–2690. PMID: 29880129.12. Powell J. Trust me, I'm a chatbot: how artificial intelligence in health care fails the turing test. J Med Internet Res. 2019; 21(10):e16222. PMID: 31661083.
Article13. Kim HS. Decision-making in artificial intelligence: is it always correct? J Korean Med Sci. 2020; 35(1):e1. PMID: 31898430.
Article14. Kim HS, Sun C, Yang SJ, Sun L, Li F, Choi IY, et al. Randomized, open-label, parallel group study to evaluate the effect of internet-based glucose management system on subject with diabetes in China. Telemed J E Health. 2016; 22(8):666–674. PMID: 26938489.15. Kim HS, Yang SJ, Jeong YJ, Kim YE, Hong SW, Cho JH. Satisfaction survey on information technology-based glucose monitoring system targeting diabetes mellitus in private local clinics in Korea. Diabetes Metab J. 2017; 41(3):213–222. PMID: 28657235.
Article16. Kim HS, Kim H, Lee S, Lee KH, Kim JH. Current clinical status of telehealth in Korea: categories, scientific basis, and obstacles. Healthc Inform Res. 2015; 21(4):244–250. PMID: 26618030.
Article17. Maeder A, Poultney N, Morgan G, Lippiatt R. Patient compliance in home-based self-care telehealth projects. J Telemed Telecare. 2015; 21(8):439–442. PMID: 26556057.
Article18. Kim HS, Choi W, Baek EK, Kim YA, Yang SJ, Choi IY, et al. Efficacy of the smartphone-based glucose management application stratified by user satisfaction. Diabetes Metab J. 2014; 38(3):204–210. PMID: 25003074.
Article19. Blakey JD, Bender BG, Dima AL, Weinman J, Safioti G, Costello RW. Digital technologies and adherence in respiratory diseases: the road ahead. Eur Respir J. 2018; 52(5):1801147. PMID: 30409819.
Article20. Dunn P, Hazzard E. Technology approaches to digital health literacy. Int J Cardiol. 2019; 293:294–296. PMID: 31350037.
Article21. Elison S, Ward J, Williams C, Espie C, Davies G, Dugdale S, et al. Feasibility of a UK community-based, eTherapy mental health service in Greater Manchester: repeated-measures and between-groups study of ‘Living Life to the Full Interactive’, ‘Sleepio’ and ‘Breaking Free Online’ at ‘Self Help Services’. BMJ Open. 2017; 7(7):e016392.
Article22. Villalva CM, Alvarez-Muiño XL, Mondelo TG, Fachado AA, Fernández JC. Adherence to treatment in hypertension. Adv Exp Med Biol. 2017; 956:129–147. PMID: 27757938.
Article23. Shahiwala A. Formulation approaches in enhancement of patient compliance to oral drug therapy. Expert Opin Drug Deliv. 2011; 8(11):1521–1529. PMID: 21995544.
Article24. Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, et al. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov. 2010; 9(3):203–214. PMID: 20168317.25. Kim HS, Kim DJ, Yoon KH. Medical big data is not yet available: why we need realism rather than exaggeration. Endocrinol Metab (Seoul). 2019; 34(4):349–354. PMID: 31884734.
Article26. Park SH, Han K. Methodologic guide for evaluating clinical performance and effect of artificial intelligence technology for medical diagnosis and prediction. Radiology. 2018; 286(3):800–809. PMID: 29309734.27. Wax PM. Elixirs, diluents, and the passage of the 1938 Federal Food, Drug and Cosmetic Act. Ann Intern Med. 1995; 122(6):456–461. PMID: 7856995.
Article28. Park SH, Do KH, Choi JI, Sim JS, Yang DM, Eo H, et al. Principles for evaluating the clinical implementation of novel digital healthcare devices. J Korean Med Assoc. 2018; 61(12):765–775.
Article29. Park SH, Kressel HY. Connecting technological innovation in artificial intelligence to real-world medical practice through rigorous clinical validation: what peer-reviewed medical journals could do. J Korean Med Sci. 2018; 33(22):e152. PMID: 29805337.
Article30. Hermsen S, Moons J, Kerkhof P, Wiekens C, De Groot M. Determinants for sustained use of an activity tracker: observational study. JMIR Mhealth Uhealth. 2017; 5(10):e164. PMID: 29084709.
Article31. Thorup C, Hansen J, Grønkjær M, Andreasen JJ, Nielsen G, Sørensen EE, et al. Cardiac patients' walking activity determined by a step counter in cardiac telerehabilitation: data from the intervention arm of a randomized controlled trial. J Med Internet Res. 2016; 18(4):e69. PMID: 27044310.
Article