J Korean Neurosurg Soc.
2020 Nov;63(6):689-697. 10.3340/jkns.2020.0056.
Effect of Pioglitazone on Perihematomal Edema in Intracerebral Hemorrhage Mouse Model by Regulating NLRP3 Expression and Energy Metabolism
- Affiliations
-
- 1Department of Neurosurgery, Bucheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Neurosurgery, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Biomedical Research Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Neurosurgery, Cell Death Disease Research Center, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2508598
- DOI: http://doi.org/10.3340/jkns.2020.0056
Abstract
Objective
: Cerebral edema is the predominant mechanism of secondary inflammation after intracerebral hemorrhage (ICH). Pioglitazone, peroxisome proliferator-activated receptor gamma agonist has been shown to play a role in regulation of central nervous system inflammation. Here, we examined the pharmacological effects of pioglitazone in an ICH mouse model and investigated its regulation on NLRP3 inflammasome and glucose metabolism.
Methods
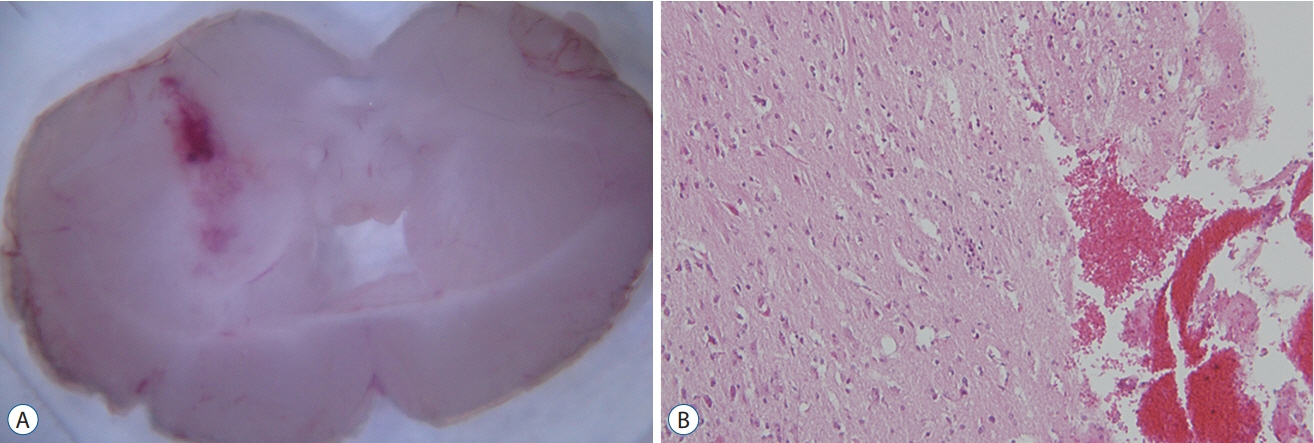
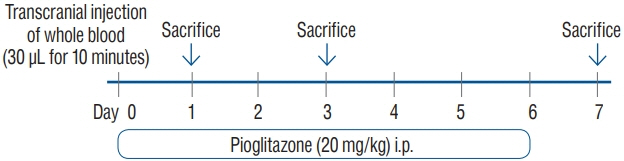
: The ICH model was established in C57 BL/6 mice by the stereotactical inoculation of blood (30 µL) into the right frontal lobe. The treatment group was administered i.p. pioglitazone (20 mg/kg) for 1, 3, and 6 days. The control group was administered i.p. phosphate-buffered saline for 1, 3, and 6 days. We investigated brain water contents, NLRP3 expression, and changes in the metabolites in the ICH model using liquid chromatography-tandem mass spectrometry.
Results
: On day 3, brain edema in the mice treated with pioglitazone was decreased more than that in the control group. Expression levels of NLRP3 in the ICH model treated with pioglitazone were decreased more than those of the control mice on days 3 and 7. The pioglitazone group showed higher levels of glycolytic metabolites than those in the ICH mice. Lactate production was increased in the ICH mice treated with pioglitazone.
Conclusion
: Our results demonstrated less brain swelling following ICH in mice treated with pioglitazone. Pioglitazone decreased NLRP3-related brain edema and increased anaerobic glycolysis, resulting in the production of lactate in the ICH mice model. NLRP3 might be a therapeutic target for ICH recovery.
Keyword
Figure
Reference
-
References
1. Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci. 30:2967–2978. 2010.
Article2. Alessandri B, Schwandt E, Kamada Y, Nagata M, Heimann A, Kempski O. The neuroprotective effect of lactate is not due to improved glutamate uptake after controlled cortical impact in rats. J Neurotrauma. 29:2181–2191. 2012.
Article3. Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 42:1781–1786. 2011.4. Au A. Metabolomics and lipidomics of ischemic stroke. Adv Clin Chem. 85:31–69. 2018.
Article5. Becatti M, Taddei N, Cecchi C, Nassi N, Nassi PA, Fiorillo C. SIRT1 modulates MAPK pathways in ischemic-reperfused cardiomyocytes. Cell Mol Life Sci. 69:2245–2260. 2012.
Article6. Berthet C, Castillo X, Magistretti PJ, Hirt L. New evidence of neuroprotection by lactate after transient focal cerebral ischaemia: extended benefit after intracerebroventricular injection and efficacy of intravenous administration. Cerebrovasc Dis. 34:329–335. 2012.
Article7. Castro MA, Beltrán FA, Brauchi S, Concha II. A metabolic switch in brain: glucose and lactate metabolism modulation by ascorbic acid. J Neurochem. 110:423–440. 2009.
Article8. Chinopoulos C. Which way does the citric acid cycle turn during hypoxia? The critical role of α-ketoglutarate dehydrogenase complex. J Neurosci Res. 91:1030–1043. 2013.
Article9. Esen F, Erdem T, Aktan D, Kalayci R, Cakar N, Kaya M, et al. Effects of magnesium administration on brain edema and blood-brain barrier breakdown after experimental traumatic brain injury in rats. J Neurosurg Anesthesiol. 15:119–125. 2003.
Article10. Fann DY, Lee SY, Manzanero S, Chunduri P, Sobey CG, Arumugam TV. Pathogenesis of acute stroke and the role of inflammasomes. Ageing Res Rev. 12:941–966. 2013.
Article11. Gallagher CN, Carpenter KL, Grice P, Howe DJ, Mason A, Timofeev I, et al. The human brain utilizes lactate via the tricarboxylic acid cycle: a 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain. 132(Pt 10):2839–2849. 2009.
Article12. Helmy MM, Helmy MW, El-Mas MM. Additive renoprotection by pioglitazone and fenofibrate against inflammatory, oxidative and apoptotic manifestations of cisplatin nephrotoxicity: modulation by PPARs. PLoS One. 10:e0142303. 2015.
Article13. Huang Q, Sun M, Li M, Zhang D, Han F, Wu JC, et al. Combination of NAD+ and NADPH offers greater neuroprotection in ischemic stroke models by relieving metabolic stress. Mol Neurobiol. 55:6063–6075. 2018.
Article14. Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 105:481–491. 2009.
Article15. Irrera N, Pizzino G, Calò M, Pallio G, Mannino F, Famà F, et al. Lack of the Nlrp3 inflammasome improves mice recovery following traumatic brain injury. Front Pharmacol. 8:459. 2017.
Article16. Izawa Y, Takahashi S, Suzuki N. Pioglitazone enhances pyruvate and lactate oxidation in cultured neurons but not in cultured astroglia. Brain Res. 1305:64–73. 2009.
Article17. Katsuki H. Exploring neuroprotective drug therapies for intracerebral hemorrhage. J Pharmacol Sci. 114:366–378. 2010.
Article18. Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 11:720–731. 2012.
Article19. Kim SJ, Kim SH, Kim JH, Hwang S, Yoo HJ. Understanding metabolomics in biomedical research. Endocrinol Metab (Seoul). 31:7–16. 2016.
Article20. Lebeaupin C, Proics E, de Bieville CH, Rousseau D, Bonnafous S, Patouraux S, et al. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 6:e1879. 2015.
Article21. Li Y, Yang J, Chen MH, Wang Q, Qin MJ, Zhang T, et al. Ilexgenin A inhibits endoplasmic reticulum stress and ameliorates endothelial dysfunction via suppression of TXNIP/NLRP3 inflammasome activation in an AMPK dependent manner. Pharmacol Res. 99:101–115. 2015.
Article22. Lu A, Tang Y, Ran R, Ardizzone TL, Wagner KR, Sharp FR. Brain genomics of intracerebral hemorrhage. J Cereb Blood Flow Metab. 26:230–252. 2006.
Article23. Mayer SA, Rincon F. Treatment of intracerebral haemorrhage. Lancet Neurol. 4:662–672. 2005.
Article24. Ribo M, Grotta JC. Latest advances in intracerebral hemorrhage. Curr Neurol Neurosci Rep. 6:17–22. 2006.
Article25. Rice AC, Zsoldos R, Chen T, Wilson MS, Alessandri B, Hamm RJ, et al. Lactate administration attenuates cognitive deficits following traumatic brain injury. Brain Res. 928:156–159. 2002.
Article26. Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 391:79–82. 1998.
Article27. Rolland WB 2nd, Manaenko A, Lekic T, Hasegawa Y, Ostrowski R, Tang J, et al. FTY720 is neuroprotective and improves functional outcomes after intracerebral hemorrhage in mice. Acta Neurochir Suppl. 111:213–217. 2011.
Article28. Rynkowski MA, Kim GH, Komotar RJ, Otten ML, Ducruet AF, Zacharia BE, et al. A mouse model of intracerebral hemorrhage using autologous blood infusion. Nat Protoc. 3:122–128. 2008.
Article29. Sahuquillo J, Merino MA, Sánchez-Guerrero A, Arikan F, Vidal-Jorge M, Martínez-Valverde T, et al. Lactate and the lactate-to-pyruvate molar ratio cannot be used as independent biomarkers for monitoring brain energetic metabolism: a microdialysis study in patients with traumatic brain injuries. PLoS One. 9:e102540. 2014.
Article30. Sánchez-Abarca LI, Tabernero A, Medina JM. Oligodendrocytes use lactate as a source of energy and as a precursor of lipids. Glia. 36:321–329. 2001.
Article31. Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 327:296–300. 2010.
Article32. Song N, Liu ZS, Xue W, Bai ZF, Wang QY, Dai J, et al. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol Cell. 68:185–197.e6. 2017.
Article33. Strbian D, Kovanen PT, Karjalainen-Lindsberg ML, Tatlisumak T, Lindsberg PJ. An emerging role of mast cells in cerebral ischemia and hemorrhage. Ann Med. 41:438–450. 2009.
Article34. Stutz A, Kolbe CC, Stahl R, Horvath GL, Franklin BS, van Ray O, et al. NLRP3 inflammasome assembly is regulated by phosphorylation of the pyrin domain. J Exp Med. 214:1725–1736. 2017.
Article35. Timofeev I, Carpenter KL, Nortje J, Al-Rawi PG, O’Connell MT, Czosnyka M, et al. Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain. 134(Pt 2):484–494. 2011.
Article36. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 324:1029–1033. 2009.
Article37. Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 92:463–477. 2010.
Article38. Wang J, Doré S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 27:894–908. 2007.
Article39. Wang J, Song MY, Lee JY, Kwon KS, Park BH. The NLRP3 inflammasome is dispensable for ER stress-induced pancreatic β-cell damage in Akita mice. Biochem Biophys Res Commun. 466:300–305. 2015.
Article40. Wang Y, Yu B, Wang L, Yang M, Xia Z, Wei W, et al. Pioglitazone ameliorates glomerular NLRP3 inflammasome activation in apolipoprotein E knockout mice with diabetes mellitus. PLoS One. 12:e0181248. 2017.
Article41. Yao ST, Cao F, Chen JL, Chen W, Fan RM, Li G, et al. NLRP3 is required for complement-mediated caspase-1 and IL-1beta activation in ICH. J Mol Neurosci. 61:385–395. 2017.
Article42. Ying W, Wei G, Wang D, Wang Q, Tang X, Shi J, et al. Intranasal administration with NAD+ profoundly decreases brain injury in a rat model of transient focal ischemia. Front Biosci. 12:2728–2734. 2007.
Article43. Zhao Y, Li Q, Zhao W, Li J, Sun Y, Liu K, et al. Astragaloside IV and cycloastragenol are equally effective in inhibition of endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in the endothelium. J Ethnopharmacol. 169:210–218. 2015.
Article44. Zhu X, Tao L, Tejima-Mandeville E, Qiu J, Park J, Garber K, et al. Plasmalemma permeability and necrotic cell death phenotypes after intracerebral hemorrhage in mice. Stroke. 43:524–531. 2012.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protracted Perihematomal Edema after Fibrinolysis Therapy with Urokinase
- Prior Use of 3-Hydroxy-3-Methyl-Glutaryl-Coenzyme A Reductase Inhibitor, Simvastatin Fails to Improve Outcome after Experimental Intracerebral Hemorrhage
- Diagnostic Usefulness of Perilesional Edema around Intracerebral Hemorrhage in Predicting Underlying Causes
- Role of CRTC2 in Metabolic Homeostasis: Key Regulator of Whole-Body Energy Metabolism?
- Luteolin Protects Cardiomyocytes Cells against Lipopolysaccharide-Induced Apoptosis and Inflammatory Damage by Modulating Nlrp3