J Korean Med Sci.
2020 Nov;35(43):e388. 10.3346/jkms.2020.35.e388.
Impact of COVID-19 Pandemic on the National PPM Tuberculosis Control Project in Korea: the Korean PPM Monitoring Database between July 2019 and June 2020
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Daejeon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea
- 4Division of Pulmonary, Allergy and Critical Care Medicine, Department of Internal Medicine, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea
- 5Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 6Division of Tuberculosis Prevention and Control, Bureau of Infectious Disease Policy, Korea Disease Control and Prevention Agency, Osong, Korea
- 7Director for Epidemiological Investigation Analysis, Director General for Public Health Emergency Preparedness, Korea Disease Control and Prevention Agency, Cheongju, Korea
- 8Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 9Division of Pulmonary Medicine, Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea
- KMID: 2508503
- DOI: http://doi.org/10.3346/jkms.2020.35.e388
Abstract
- Background
The coronavirus disease 2019 (COVID-19) pandemic caused disruptions to healthcare systems and endangered the control and prevention of tuberculosis (TB). We investigated the nationwide effects of COVID-19 on the national Public-Private Mix (PPM) TB control project in Korea, using monitoring indicators from the Korean PPM monitoring database.
Methods
The Korean PPM monitoring database includes data from patients registered at PPM hospitals throughout the country. Data of six monitoring indicators for active TB cases updated between July 2019 and June 2020 were collected. The data of each cohort throughout the country and in Daegu-Gyeongbuk, Seoul Metropolitan Area, and Jeonnam-Jeonbuk were collated to provide nationwide data. The data were compared using the χ 2 test for trend to evaluate quarterly trends of each monitoring indicator at the national level and in the prespecified regions.
Results
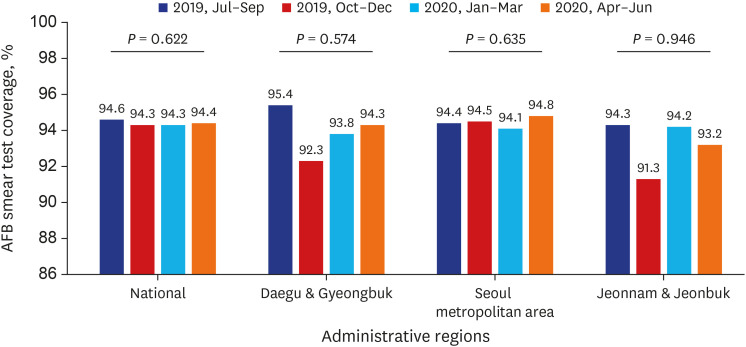
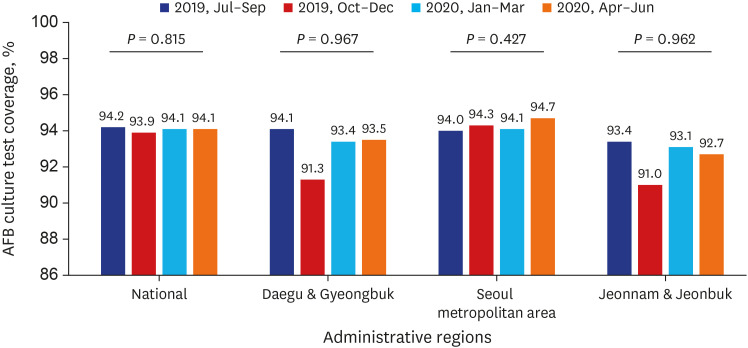
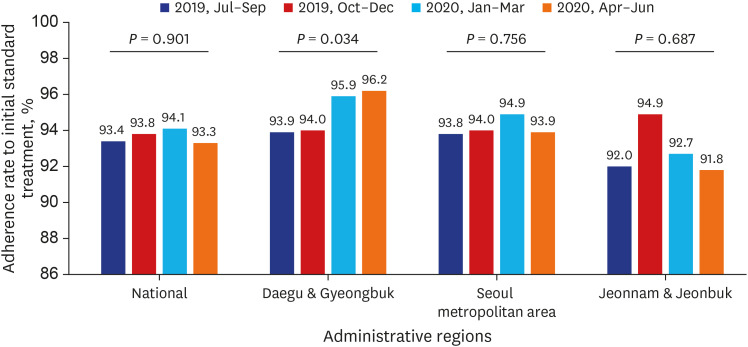
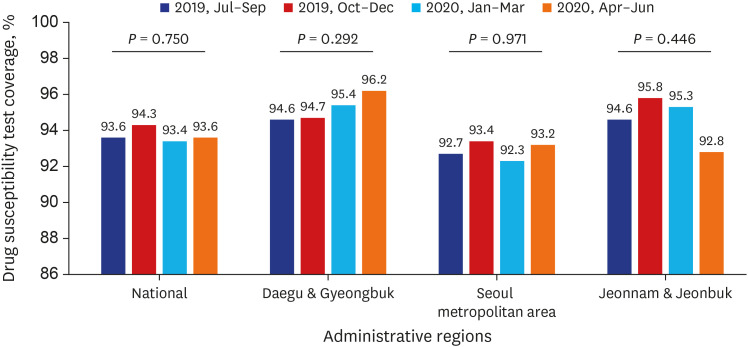
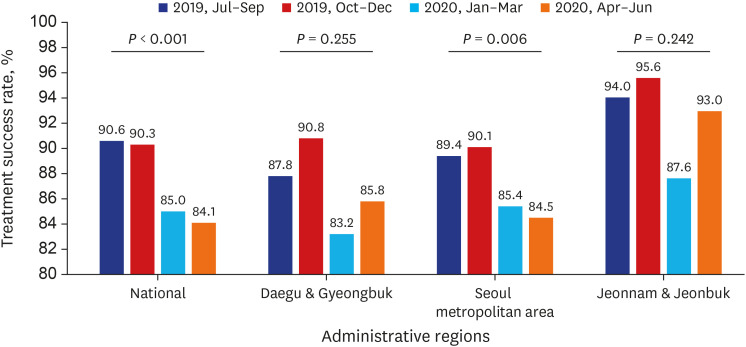
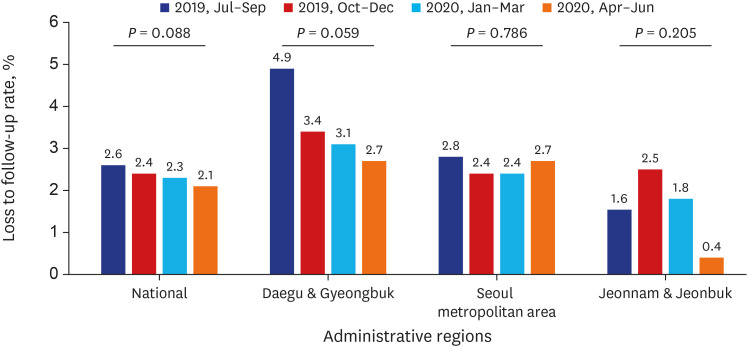
Test coverages of sputum smear (P = 0.622) and culture (P = 0.815), drug susceptibility test (P = 0.750), and adherence rate to initial standard treatment (P = 0.901) at the national level were not significantly different during the study period. The rate of loss to follow-up among TB cases at the national level was not significantly different (P = 0.088) however, the treatment success rate among the smear-positive drug-susceptible pulmonary TB cohort at the national level significantly decreased, from 90.6% to 84.1% (P < 0.001). Treatment success rate in the Seoul metropolitan area also significantly decreased during the study period, from 89.4% to 84.5% (P = 0.006).
Conclusion
Our study showed that initial TB management during the COVID-19 pandemic was properly administered under the PPM project in Korea. However, our study cannot confirm or conclude a decreased treatment success rate after the COVID-19 pandemic due to limited data.
Keyword
Figure
Cited by 2 articles
-
Increased Healthcare Delays in Tuberculosis Patients During the First Wave of COVID-19 Pandemic in Korea: A Nationwide Cross-Sectional Study
Jinsoo Min, Yousang Ko, Hyung Woo Kim, Hyeon-Kyoung Koo, Jee Youn Oh, Yun-Jeong Jeong, Hyeon Hui Kang, Kwang Joo Park, Yong Il Hwang, Jin Woo Kim, Joong Hyun Ahn, Yangjin Jegal, Ji Young Kang, Sung-Soon Lee, Jae Seuk Park, Ju Sang Kim
J Korean Med Sci. 2021;37(3):e20. doi: 10.3346/jkms.2022.37.e20.Impact of the Coronavirus Disease Pandemic on Patients with Head Injuries in South Korea
Taek Min Nam, Do-Hyung Kim, Ji Hwan Jang, Young Zoon Kim, Kyu Hong Kim, Seung Hwan Kim
J Korean Neurosurg Soc. 2022;65(2):269-275. doi: 10.3340/jkns.2021.0076.
Reference
-
1. World Health Organization. Coronavirus disease 2019 (COVID-19) situation report-51 (11 March 2020). Accessed October 27, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10.2. Chudasama YV, Gillies CL, Zaccardi F, Coles B, Davies MJ, Seidu S, et al. Impact of COVID-19 on routine care for chronic diseases: a global survey of views from healthcare professionals. Diabetes Metab Syndr. 2020; 14(5):965–967. PMID: 32604016.
Article3. Alene KA, Wangdi K, Clements ACA. Impact of the COVID-19 pandemic on tuberculosis control: an overview. Trop Med Infect Dis. 2020; 5(3):123.
Article4. McQuaid CF, McCreesh N, Read JM, Sumner T. CMMID COVID-19 Working Group, Houben RMGJ, et al. The potential impact of COVID-19-related disruption on tuberculosis burden. Eur Respir J. 2020; 56(2):2001718. PMID: 32513784.
Article5. Oh J, Lee JK, Schwarz D, Ratcliffe HL, Markuns JF, Hirschhorn LR. National response to COVID-19 in the Republic of Korea and lessons learned for other countries. Health Syst Reform. 2020; 6(1):e1753464. PMID: 32347772.
Article6. Kwak N, Hwang SS, Yim JJ. Effect of COVID-19 on tuberculosis notification, South Korea. Emerg Infect Dis. 2020; 26(10):2506–2508. PMID: 32672531.
Article7. Min J, Kim HW, Ko Y, Oh JY, Kang JY, Lee J, et al. Tuberculosis surveillance and monitoring under the National Public-Private Mix Tuberculosis Control Project in South Korea 2016-2017. Tuberc Respir Dis (Seoul). 2020; 83(3):218–227. PMID: 32610836.
Article8. Korea Centers for Disease Control & Prevention. Annual Report on the Notified Tuberculosis in Korea, 2019. Cheongju, Korea: Korea Centers for Disease Control & Prevention;2020.9. Kang HY, Yoo H, Park W, Go U, Jeong E, Jung KS, et al. Tuberculosis Notification Completeness and Timeliness in the Republic of Korea During 2012-2014. Osong Public Health Res Perspect. 2016; 7(5):320–326. PMID: 27812491.
Article10. World Health Organization. Definitions and Reporting Framework for Tuberculosis – 2013 Revision. Geneva, Switzerland: World Health Organization;2013.11. Korea Centers for Disease Control & Prevention. Korean Guidelines for Tuberculosis. 4th ed. Cheongju, Korea: Joint Committee for the Revision of Korean Guidelines for Tuberculosis and Korea Centers for Disease Control & Prevention;2020.12. Kim M, Park K, Kim Y, Kim Y, Yeom H, Hwang I, et al. Weekly report on the COVID-19 situation in the Republic of Korea (as of July 4, 2020). Public Health Wkly Rep. 2020; 13(28):2032–2046.13. World Health Organization. COVID-19: considerations for tuberculosis (TB) care. Accessed 24 Aug 2020. https://www.who.int/docs/default-source/documents/tuberculosis/infonote-tb-covid-19.pdf.14. Magassouba AS, Diallo BD, Camara LM, Sow K, Camara S, Bah B, et al. Impact of the Ebola virus disease outbreak (2014–2016) on tuberculosis surveillance activities by Guinea's National Tuberculosis Control Program: a time series analysis. BMC Public Health. 2020; 20(1):1200. PMID: 32753044.
Article15. Desta KT, Kessely DB, Daboi JG. Evaluation of the performance of the National Tuberculosis Program of Liberia during the 2014–2015 Ebola outbreak. BMC Public Health. 2019; 19(1):1221. PMID: 31484578.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increasing the Treatment Success Rate of Tuberculosis in a Private Hospital through Public-Private Mix (PPM) Project
- COVID-19 from the Perspective of a Gastroenterologist
- The impact of the COVID-19 pandemic on in-hospital mortality in patients admitted through the emergency department
- Impact of the COVID-19 Pandemic on Gastric Cancer Screening in South Korea: Results From the Korean National Cancer Screening Survey (2017–2021)
- COVID-19 and Fungal Infection