J Korean Med Sci.
2020 Nov;35(42):e346. 10.3346/jkms.2020.35.e346.
Clinical Outcomes of Early Extubation Strategy in Patients Undergoing Extracorporeal Membrane Oxygenation as a Bridge to Heart Transplantation
- Affiliations
-
- 1Division of Cardiology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Thoracic and Cardiovascular Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Critical Care Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2508427
- DOI: http://doi.org/10.3346/jkms.2020.35.e346
Abstract
- Background
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) might be considered a bridge therapy in patients who are expected to have short waiting times for heart transplantation. We investigated the clinical outcomes of patients who underwent VA-ECMO as a bridge to heart transplantation and whether the deployment of an early extubation ECMO strategy is beneficial.
Methods
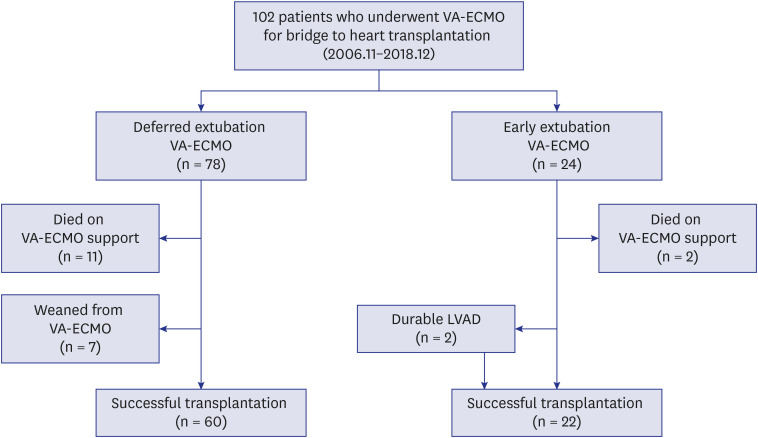
Between November 2006 and December 2018, we studied 102 patients who received VA-ECMO as a bridge to heart transplantation. We classified these patients into an early extubation ECMO group (n = 24) and a deferred extubation ECMO group (n = 78) based on the length of the intubated period on VA-ECMO (≤ 48 hours or > 48 hours). The primary outcome was in-hospital mortality.
Results
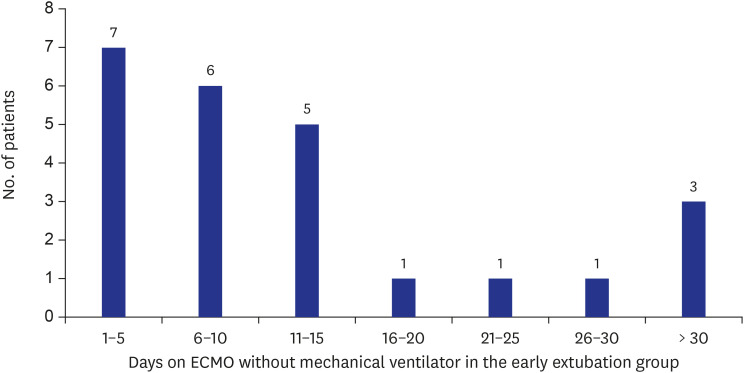
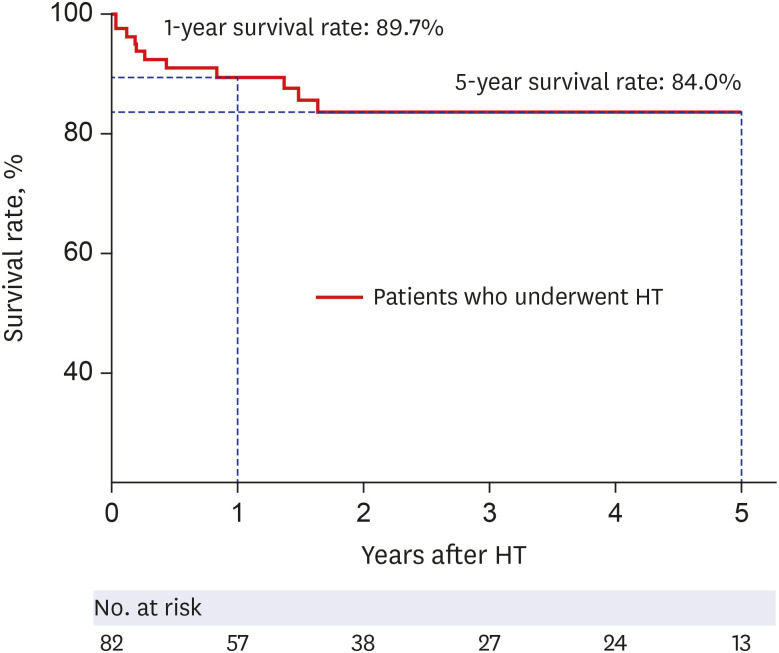
The median duration of early extubation VA-ECMO was 10.0 (4.3–17.3) days. The most common cause for patients to be put on ECMO was dilated cardiomyopathy (65.7%) followed by ischemic cardiomyopathy (11.8%). In-hospital mortality rates for the deferred extubation and early extubation groups, respectively, were 24.4% and 8.3% (P = 0.147). During the study period, in the deferred extubation group, 60 (76.9%) underwent transplantation, while 22 (91.7%) underwent transplantation in the early extubation group. Delirium occurred in 83.3% and 33.3% of patients from the deferred extubation and early extubation groups (P < 0.001) and microbiologically confirmed infection was identified in 64.1% and 41.7% of patients from the two groups (P = 0.051), respectively.
Conclusion
VA-ECMO as a bridge therapy seems to be feasible for deployment in patients with a short waiting time for heart transplantation. Deployment of the early extubation ECMO strategy was associated with reductions in delirium and infection in this population.
Keyword
Figure
Cited by 1 articles
-
Association between a Multidisciplinary Team Approach and Clinical Outcomes in Patients Undergoing Extracorporeal Cardiopulmonary Resuscitation in the Emergency Department
Ji Han Lee, Ryoung Eun Ko, Taek Kyu Park, Yang Hyun Cho, Gee Young Suh, Jeong Hoon Yang
Korean Circ J. 2021;51(11):908-918. doi: 10.4070/kcj.2021.0167.
Reference
-
1. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017; 136(6):e137–61. PMID: 28455343.
Article2. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016; 37(27):2129–2200. PMID: 27206819.3. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011; 8(1):30–41. PMID: 21060326.
Article4. Chaudhry SP, Stewart GC. Advanced heart failure: prevalence, natural history, and prognosis. Heart Fail Clin. 2016; 12(3):323–333. PMID: 27371510.5. Lund LH, Edwards LB, Dipchand AI, Goldfarb S, Kucheryavaya AY, Levvey BJ, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-third adult heart transplantation report-2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant. 2016; 35(10):1158–1169. PMID: 27772668.
Article6. Kirklin JK, Cantor R, Mohacsi P, Gummert J, De By T, Hannan MM, et al. First annual IMACS report: a global International Society for Heart and Lung Transplantation Registry for Mechanical Circulatory Support. J Heart Lung Transplant. 2016; 35(4):407–412. PMID: 26922275.
Article7. Barge-Caballero E, Almenar-Bonet L, Gonzalez-Vilchez F, Lambert-Rodríguez JL, González-Costello J, Segovia-Cubero J, et al. Clinical outcomes of temporary mechanical circulatory support as a direct bridge to heart transplantation: a nationwide Spanish registry. Eur J Heart Fail. 2018; 20(1):178–186. PMID: 28949079.
Article8. Werdan K, Gielen S, Ebelt H, Hochman JS. Mechanical circulatory support in cardiogenic shock. Eur Heart J. 2014; 35(3):156–167. PMID: 24014384.
Article9. Park C, Jee YH, Jung KJ. Age-period-cohort analysis of the suicide rate in Korea. J Affect Disord. 2016; 194:16–20. PMID: 26802502.
Article10. Langer T, Santini A, Bottino N, Crotti S, Batchinsky AI, Pesenti A, et al. “Awake” extracorporeal membrane oxygenation (ECMO): pathophysiology, technical considerations, and clinical pioneering. Crit Care. 2016; 20(1):150. PMID: 27357690.
Article11. Wu J, Huang M. How do we maximize the humanistic care of a long-range (36-day) venoarterial extracorporeal membrane oxygenation and successfully bridged to heart transplantation. J Clin Anesth. 2019; 58:105–106. PMID: 31151039.
Article12. Stevenson LW, Pagani FD, Young JB, Jessup M, Miller L, Kormos RL, et al. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant. 2009; 28(6):535–541. PMID: 19481012.
Article13. Park TK, Yang JH, Choi SH, Song YB, Hahn JY, Choi JH, et al. Clinical impact of intra-aortic balloon pump during extracorporeal life support in patients with acute myocardial infarction complicated by cardiogenic shock. BMC Anesthesiol. 2014; 14(1):27. PMID: 24725532.
Article14. Lang G, Kim D, Aigner C, Matila J, Taghavi S, Jaksch P, et al. Awake extracorporeal membrane oxygenation bridging for pulmonary retransplantation provides comparable results to elective retransplantation. J Heart Lung Transplant. 2014; 33(12):1264–1272. PMID: 25169957.
Article15. Fuehner T, Kuehn C, Hadem J, Wiesner O, Gottlieb J, Tudorache I, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med. 2012; 185(7):763–768. PMID: 22268135.
Article16. Crotti S, Iotti GA, Lissoni A, Belliato M, Zanierato M, Chierichetti M, et al. Organ allocation waiting time during extracorporeal bridge to lung transplant affects outcomes. Chest. 2013; 144(3):1018–1025. PMID: 23599162.
Article17. Olsson KM, Simon A, Strueber M, Hadem J, Wiesner O, Gottlieb J, et al. Extracorporeal membrane oxygenation in nonintubated patients as bridge to lung transplantation. Am J Transplant. 2010; 10(9):2173–2178. PMID: 20636463.
Article18. Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016; 63(5):e61–111. PMID: 27418577.19. Guenther U, Popp J, Koecher L, Muders T, Wrigge H, Ely EW, et al. Validity and reliability of the CAM-ICU flowsheet to diagnose delirium in surgical ICU patients. J Crit Care. 2010; 25(1):144–151. PMID: 19828283.
Article20. Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315(8):762–774. PMID: 26903335.21. Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant. 2014; 33(6):555–564. PMID: 24856259.
Article22. Aleksova N, Chih S. The role of durable left ventricular assist devices in advanced heart failure: would my patient benefit? Can J Cardiol. 2017; 33(4):540–543. PMID: 28159376.
Article23. Urban M, Pirk J, Dorazilova Z, Netuka I. How does successful bridging with ventricular assist device affect cardiac transplantation outcome? Interact Cardiovasc Thorac Surg. 2011; 13(4):405–409. PMID: 21788304.
Article24. Baras Shreibati J, Goldhaber-Fiebert JD, Banerjee D, Owens DK, Hlatky MA. Cost-effectiveness of left ventricular assist devices in ambulatory patients with advanced heart failure. JACC Heart Fail. 2017; 5(2):110–119. PMID: 28017351.
Article25. Patel N, Kalra R, Doshi R, Bajaj NS, Arora G, Arora P. Trends and cost of heart transplantation and left ventricular assist devices: impact of proposed federal cuts. JACC Heart Fail. 2018; 6(5):424–432. PMID: 29724365.26. Poptsov V, Spirina E, Dogonasheva A, Zolotova E. Five years' experience with a peripheral veno-arterial ECMO for mechanical bridge to heart transplantation. J Thorac Dis. 2019; 11(Suppl 6):S889–S901. PMID: 31183168.
Article27. Chung JC, Tsai PR, Chou NK, Chi NH, Wang SS, Ko WJ. Extracorporeal membrane oxygenation bridge to adult heart transplantation. Clin Transplant. 2010; 24(3):375–380. PMID: 19744095.
Article28. Kass M, Moon M, Vo M, Singal R, Ravandi A. Awake extracorporeal membrane oxygenation for very high-risk coronary angioplasty. Can J Cardiol. 2015; 31(2):227.e11–227.e13.
Article29. Liu S, Ravandi A, Kass M, Elbarouni B. A case of awake percutaneous extracorporeal membrane oxygenation for high-risk percutaneous coronary intervention. Cureus. 2017; 9(4):e1191. PMID: 28553569.
Article30. Kacer J, Lindovska M, Surovcik R, Netuka I, Mlejnsky F, Grus T, et al. Refractory cardiogenic shock due to extensive anterior STEMI with covered left ventricular free wall rupture treated with awake VA-ECMO and LVAD as a double bridge to heart transplantation - collaboration of three cardiac centres. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015; 159(4):681–687. PMID: 26498212.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Awakening in extracorporeal membrane oxygenation as a bridge to lung transplantation
- Extracorporeal Membrane Oxygenation for 67 Days as a Bridge to Heart Transplantation in a Postcardiotomy Patient with Failing Heart and Mediastinitis
- Outcomes of Extracorporeal Membrane Oxygenation in Children: An 11-Year Single-Center Experience in Korea
- Delayed Repair of Ventricular Septal Rupture Following Preoperative Awake Extracorporeal Membrane Oxygenation Support
- A case of rescuing a patient with acute cardiovascular instability from sudden and massive intraoperative pulmonary thromboembolism by extracorporeal membrane oxygenation