Yeungnam Univ J Med.
2020 Oct;37(4):269-276. 10.12701/yujm.2020.00458.
Therapeutic potential of targeting kinase inhibition in patients with idiopathic pulmonary fibrosis
- Affiliations
-
- 1Smart-Ageing Convergence Research Center, Yeungnam University College of Medicine, Daegu, Korea
- 2Department of Pharmacology, Yeungnam University College of Medicine, Daegu, Korea
- 3Department of Microbiology, Ewha Womans University College of Medicine, Seoul, Korea
- KMID: 2508171
- DOI: http://doi.org/10.12701/yujm.2020.00458
Abstract
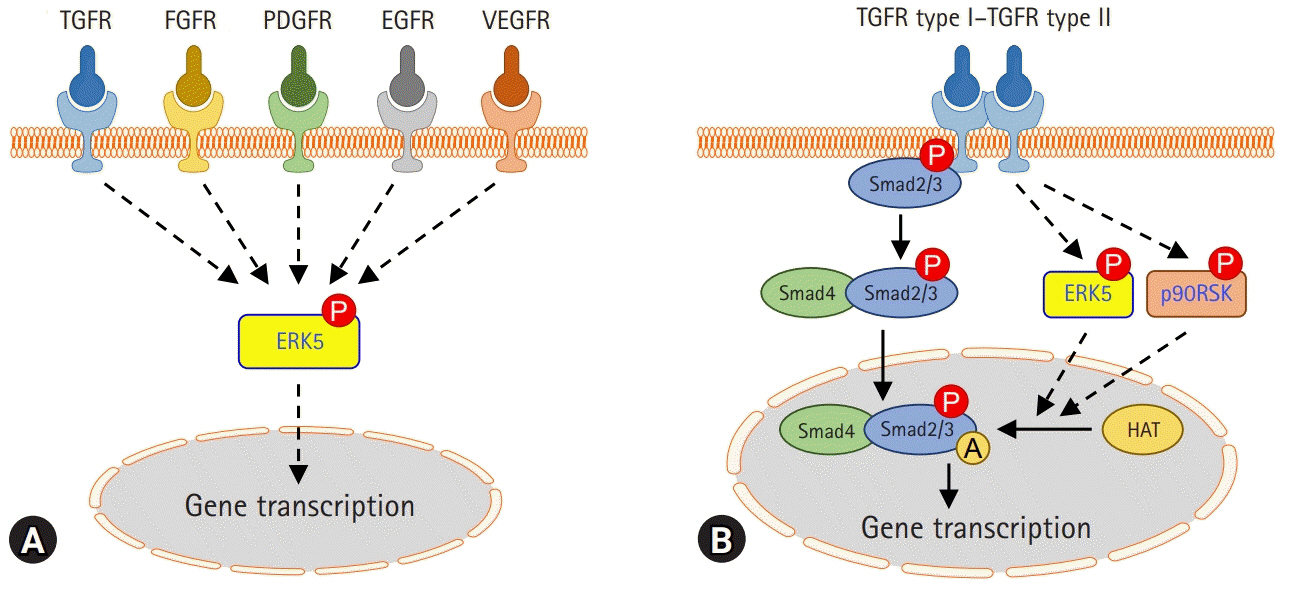
- Fibrosis is characterized by excessive accumulation of extracellular matrix components. The fibrotic process ultimately leads to organ dysfunction and failure in chronic inflammatory and metabolic diseases such as pulmonary fibrosis, advanced kidney disease, and liver cirrhosis. Idiopathic pulmonary fibrosis (IPF) is a common form of progressive and chronic interstitial lung disease of unknown etiology. Pathophysiologically, the parenchyma of the lung alveoli, interstitium, and capillary endothelium becomes scarred and stiff, which makes breathing difficult because the lungs have to work harder to transfer oxygen and carbon dioxide between the alveolar space and bloodstream. The transforming growth factor beta (TGF-) signaling pathway plays an important role in the pathogenesis of pulmonary fibrosis and scarring of the lung tissue. Recent clinical trials focused on the development of pharmacological agents that either directly or indirectly target kinases for the treatment of IPF. Therefore, to develop therapeutic targets for pulmonary fibrosis, it is essential to understand the key factors involved in the pathogenesis of pulmonary fibrosis and the underlying signaling pathway. The objective of this review is to discuss the role of kinase signaling cascades in the regulation of either TGF--dependent or other signaling pathways, including Rho-associated coiled-coil kinase, c-jun N-terminal kinase, extracellular signal-regulated kinase 5, and p90 ribosomal S6 kinase pathways, and potential therapeutic targets in IPF.
Figure
Cited by 1 articles
-
Advances in the science and treatment of respiratory diseases
Jin Hong Chung
Yeungnam Univ J Med. 2020;37(4):251-252. doi: 10.12701/yujm.2020.00661.
Reference
-
References
1. Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014; 15:786–801.
Article2. Iredale JP, Thompson A, Henderson NC. Extracellular matrix degradation in liver fibrosis: biochemistry and regulation. Biochim Biophys Acta. 2013; 1832:876–83.
Article3. Borthwick LA, Wynn TA, Fisher AJ. Cytokine mediated tissue fibrosis. Biochim Biophys Acta. 2013; 1832:1049–60.
Article4. Liu RM, Gaston Pravia KA. Oxidative stress and glutathione in TGF-beta-mediated fibrogenesis. Free Radic Biol Med. 2010; 48:1–15.5. Rockey DC, Bell PD, Hill JA. Fibrosis: a common pathway to organ injury and failure. N Engl J Med. 2015; 372:1138–49.
Article6. King TE Jr. Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med. 2005; 172:268–79.
Article7. Strongman H, Kausar I, Maher TM. Incidence, prevalence, and survival of patients with idiopathic pulmonary fibrosis in the UK. Adv Ther. 2018; 35:724–36.
Article8. Hopkins RB, Burke N, Fell C, Dion G, Kolb M. Epidemiology and survival of idiopathic pulmonary fibrosis from national data in Canada. Eur Respir J. 2016; 48:187–95.
Article9. Raghu G. Idiopathic pulmonary fibrosis: lessons from clinical trials over the past 25 years. Eur Respir J. 2017; 50:1701209.10. Noble PW, Homer RJ. Idiopathic pulmonary fibrosis: new insights into pathogenesis. Clin Chest Med. 2004; 25:749–58.
Article11. Thannickal VJ, Toews GB, White ES, Lynch JP 3rd, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004; 55:395–417.
Article12. Saito A, Horie M, Nagase T. TGF-β signaling in lung health and disease. Int J Mol Sci. 2018; 19:2460.
Article13. Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004; 85:47–64.
Article14. Nakerakanti S, Trojanowska M. The role of TGF-β receptors in fibrosis. Open Rheumatol J. 2012; 6:156–62.
Article15. Gordon KJ, Blobe GC. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta. 2008; 1782:197–228.
Article16. Hoyt DG, Lazo JS. Alterations in pulmonary mRNA encoding procollagens, fibronectin and transforming growth factor-beta precede bleomycin-induced pulmonary fibrosis in mice. J Pharmacol Exp Ther. 1988; 246:765–71.17. Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A. 1991; 88:6642–6.
Article18. Wu CF, Chiang WC, Lai CF, Chang FC, Chen YT, Chou YH, et al. Transforming growth factor β-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am J Pathol. 2013; 182:118–31.
Article19. Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Yu, Pierschbacher MD, et al. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992; 360:361–4.
Article20. Isaka Y, Tsujie M, Ando Y, Nakamura H, Kaneda Y, Imai E, et al. Transforming growth factor-beta 1 antisense oligodeoxynucleotides block interstitial fibrosis in unilateral ureteral obstruction. Kidney Int. 2000; 58:1885–92.21. Takabatake Y, Isaka Y, Mizui M, Kawachi H, Shimizu F, Ito T, et al. Exploring RNA interference as a therapeutic strategy for renal disease. Gene Ther. 2005; 12:965–73.
Article22. Zhao J, Shi W, Wang YL, Chen H, Bringas P Jr, Datto MB, et al. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2002; 282:L585–93.23. Zi Z, Chapnick DA, Liu X. Dynamics of TGF-β/Smad signaling. FEBS Lett. 2012; 586:1921–8.
Article24. Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009; 19:128–39.25. Bringardner BD, Baran CP, Eubank TD, Marsh CB. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2008; 10:287–301.
Article26. Selman M, Pardo A. Idiopathic pulmonary fibrosis: an epithelial/fibroblastic cross-talk disorder. Respir Res. 2002; 3:3.
Article27. Sakai N, Tager AM. Fibrosis of two: epithelial cell-fibroblast interactions in pulmonary fibrosis. Biochim Biophys Acta. 2013; 1832:911–21.
Article28. Camelo A, Dunmore R, Sleeman MA, Clarke DL. The epithelium in idiopathic pulmonary fibrosis: breaking the barrier. Front Pharmacol. 2014; 4:173.
Article29. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014; 370:2071–82.
Article30. Ogura T, Taniguchi H, Azuma A, Inoue Y, Kondoh Y, Hasegawa Y, et al. Safety and pharmacokinetics of nintedanib and pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2015; 45:1382–92.
Article31. Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010; 35:821–9.
Article32. Gurujeyalakshmi G, Hollinger MA, Giri SN. Pirfenidone inhibits PDGF isoforms in bleomycin hamster model of lung fibrosis at the translational level. Am J Physiol. 1999; 276:L311–8.33. Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther. 1999; 291:367–73.34. Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011; 377:1760–9.
Article35. Lopez-de la Mora DA, Sanchez-Roque C, Montoya-Buelna M, Sanchez-Enriquez S, Lucano-Landeros S, Macias-Barragan J, et al. Role and new insights of pirfenidone in fibrotic diseases. Int J Med Sci. 2015; 12:840–7.
Article36. King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014; 370:2083–92.
Article37. Jiang C, Huang H, Liu J, Wang Y, Lu Z, Xu Z. Adverse events of pirfenidone for the treatment of pulmonary fibrosis: a meta-analysis of randomized controlled trials. PLoS One. 2012; 7:e47024.
Article38. Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, Stowasser S, et al. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2015; 45:1434–45.
Article39. Hostettler KE, Zhong J, Papakonstantinou E, Karakiulakis G, Tamm M, Seidel P, et al. Anti-fibrotic effects of nintedanib in lung fibroblasts derived from patients with idiopathic pulmonary fibrosis. Respir Res. 2014; 15:157.
Article40. Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther. 2014; 349:209–20.
Article41. Richeldi L, Cottin V, Flaherty KR, Kolb M, Inoue Y, Raghu G, et al. Design of the INPULSIS™ trials: two phase 3 trials of nintedanib in patients with idiopathic pulmonary fibrosis. Respir Med. 2014; 108:1023–30.
Article42. Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016; 12:325–38.
Article43. Choi ME. Mechanism of transforming growth factor-β1 signaling: role of the mitogen-activated protein kinase. Kidney Int. 2000; 58(Suppl 77):S53–8.
Article44. Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006; 119(Pt 23):4803–10.
Article45. Yue X, Shan B, Lasky JA. TGF-β: titan of lung fibrogenesis. Curr Enzym Inhib. 2010; 6:10.2174/10067.46. Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007; 117:557–67.
Article47. Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003; 425:577–84.
Article48. Das F, Ghosh-Choudhury N, Venkatesan B, Li X, Mahimainathan L, Choudhury GG. Akt kinase targets association of CBP with SMAD 3 to regulate TGFbeta-induced expression of plasminogen activator inhibitor-1. J Cell Physiol. 2008; 214:513–27.49. Munger JS, Huang X, Kawakatsu H, Griffiths MJD, Dalton SL, Wu J, et al. The integrin αvβ6 binds and activates latent TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999; 96:319–28.
Article50. Mackinnon AC, Gibbons MA, Farnworth SL, Leffler H, Nilsson UJ, Delaine T, et al. Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. Am J Respir Crit Care Med. 2012; 185:537–46.
Article51. Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999; 20:189–206.
Article52. Kondo S, Kubota S, Shimo T, Nishida T, Yosimichi G, Eguchi T, et al. Connective tissue growth factor increased by hypoxia may initiate angiogenesis in collaboration with matrix metalloproteinases. Carcinogenesis. 2002; 23:769–76.
Article53. Shimo T, Nakanishi T, Nishida T, Asano M, Sasaki A, Kanyama M, et al. Involvement of CTGF, a hypertrophic chondrocyte-specific gene product, in tumor angiogenesis. Oncology. 2001; 61:315–22.
Article54. Takigawa M. CTGF/Hcs24 as a multifunctional growth factor for fibroblasts, chondrocytes and vascular endothelial cells. Drug News Perspect. 2003; 16:11–21.
Article55. Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell. 1993; 4:637–45.
Article56. Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996; 7:469–80.57. Shimo T, Kubota S, Kondo S, Nakanishi T, Sasaki A, Mese H, et al. Connective tissue growth factor as a major angiogenic agent that is induced by hypoxia in a human breast cancer cell line. Cancer Lett. 2001; 174:57–64.
Article58. Suzuma K, Naruse K, Suzuma I, Takahara N, Ueki K, Aiello LP, et al. Vascular endothelial growth factor induces expression of connective tissue growth factor via KDR, Flt1, and phosphatidylinositol 3-kinase-akt-dependent pathways in retinal vascular cells. J Biol Chem. 2000; 275:40725–31.
Article59. Lau LF. Cell surface receptors for CCN proteins. J Cell Commun Signal. 2016; 10:121–7.
Article60. Wang Q, Usinger W, Nichols B, Gray J, Xu L, Seeley TW, et al. Cooperative interaction of CTGF and TGF-β in animal models of fibrotic disease. Fibrogenesis Tissue Repair. 2011; 4:4.
Article61. Kono M, Nakamura Y, Suda T, Kato M, Kaida Y, Hashimoto D, et al. Plasma CCN2 (connective tissue growth factor; CTGF) is a potential biomarker in idiopathic pulmonary fibrosis (IPF). Clin Chim Acta. 2011; 412:2211–5.
Article62. Pan LH, Yamauchi K, Uzuki M, Nakanishi T, Takigawa M, Inoue H, et al. Type II alveolar epithelial cells and interstitial fibroblasts express connective tissue growth factor in IPF. Eur Respir J. 2001; 17:1220–7.
Article63. Gabbiani G. Modulation of fibroblastic cytoskeletal features during wound healing and fibrosis. Pathol Res Pract. 1994; 190:851–3.
Article64. Sandbo N, Dulin N. Actin cytoskeleton in myofibroblast differentiation: ultrastructure defining form and driving function. Transl Res. 2011; 158:181–96.
Article65. Knipe RS, Tager AM, Liao JK. The Rho kinases: critical mediators of multiple profibrotic processes and rational targets for new therapies for pulmonary fibrosis. Pharmacol Rev. 2015; 67:103–17.
Article66. Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, et al. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013; 123:1096–108.
Article67. Griffith DE, Eagle G, Thomson R, Aksamit TR, Hasegawa N, Morimoto K, et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT): a prospective, open-label, randomized study. Am J Respir Crit Care Med. 2018; 198:1559–69.
Article68. Yoshida K, Kuwano K, Hagimoto N, Watanabe K, Matsuba T, Fujita M, et al. MAP kinase activation and apoptosis in lung tissues from patients with idiopathic pulmonary fibrosis. J Pathol. 2002; 198:388–96.
Article69. Alcorn JF, van der Velden J, Brown AL, McElhinney B, Irvin CG, Janssen-Heininger YM. c-Jun N-terminal kinase 1 is required for the development of pulmonary fibrosis. Am J Respir Cell Mol Biol. 2009; 40:422–32.
Article70. Van der Velden JL, Alcorn JF, Chapman DG, Lundblad LK, Irvin CG, Davis RJ, et al. Airway epithelial specific deletion of Jun-N-terminal kinase 1 attenuates pulmonary fibrosis in two independent mouse models. PLoS One. 2020; 15:e0226904.
Article71. Bennett B, Blease K, Ye Y, Azaryan A, Ramirez-Valle F, Ceres R, et al. CC-90001, a second generation Jun N-terminal kinase (JNK) inhibitor for the treatment of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2017; 195:A5409.72. Van der Velden JL, Ye Y, Nolin JD, Hoffman SM, Chapman DG, Lahue KG, et al. JNK inhibition reduces lung remodeling and pulmonary fibrotic systemic markers. Clin Transl Med. 2016; 5:36.73. Clement DL, Mally S, Stock C, Lethan M, Satir P, Schwab A, et al. PDGFRα signaling in the primary cilium regulates NHE1-dependent fibroblast migration via coordinated differential activity of MEK1/2-ERK1/2-p90RSK and AKT signaling pathways. J Cell Sci. 2013; 126(Pt 4):953–65.
Article74. Delehedde M, Seve M, Sergeant N, Wartelle I, Lyon M, Rudland PS, et al. Fibroblast growth factor-2 stimulation of p42/44MAPK phosphorylation and IkappaB degradation is regulated by heparan sulfate/heparin in rat mammary fibroblasts. J Biol Chem. 2000; 275:33905–10.75. Seko Y, Takahashi N, Tobe K, Ueki K, Kadowaki T, Yazaki Y. Vascular endothelial growth factor (VEGF) activates Raf-1, mitogen-activated protein (MAP) kinases, and S6 kinase (p90rsk) in cultured rat cardiac myocytes. J Cell Physiol. 1998; 175:239–46.
Article76. Rovida E, Navari N, Caligiuri A, Dello Sbarba P, Marra F. ERK5 differentially regulates PDGF-induced proliferation and migration of hepatic stellate cells. J Hepatol. 2008; 48:107–15.
Article77. Roberts OL, Holmes K, Muller J, Cross DA, Cross MJ. ERK5 and the regulation of endothelial cell function. Biochem Soc Trans. 2009; 37(Pt 6):1254–9.
Article78. Kimura TE, Jin J, Zi M, Prehar S, Liu W, Oceandy D, et al. Targeted deletion of the extracellular signal-regulated protein kinase 5 attenuates hypertrophic response and promotes pressure overload-induced apoptosis in the heart. Circ Res. 2010; 106:961–70.
Article79. Urushihara M, Takamatsu M, Shimizu M, Kondo S, Kinoshita Y, Suga K, et al. ERK5 activation enhances mesangial cell viability and collagen matrix accumulation in rat progressive glomerulonephritis. Am J Physiol Renal Physiol. 2010; 298:F167–76.
Article80. Kim S, Lim JH, Woo CH. ERK5 inhibition ameliorates pulmonary fibrosis via regulating Smad3 acetylation. Am J Pathol. 2013; 183:1758–68.
Article81. Frodin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol. 1999; 151:65–77.
Article82. Morales-Ibanez O, Affo S, Rodrigo-Torres D, Blaya D, Millan C, Coll M, et al. Kinase analysis in alcoholic hepatitis identifies p90RSK as a potential mediator of liver fibrogenesis. Gut. 2016; 65:840–51.
Article83. Jo E, Park SJ, Choi YS, Jeon WK, Kim BC. Kaempferol suppresses transforming growth factor-β1-induced epithelial-to-mesenchymal transition and migration of A549 lung cancer cells by inhibiting akt1-mediated phosphorylation of Smad3 at threonine-179. Neoplasia. 2015; 17:525–37.
Article84. Kim S, Han JH, Kim S, Lee H, Kim JR, Lim JH, et al. p90RSK inhibition ameliorates TGF-β1 signaling and pulmonary fibrosis by inhibiting Smad3 transcriptional activity. Cell Physiol Biochem. 2020; 54:195–210.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pharmacological treatment of idiopathic pulmonary fibrosis and fibrosing interstitial lung diseases: current trends and future directions
- Two cases of idiopathic pulmonary fibrosis
- Idiopathic Pulmonary Fibrosis and Nonspecific Interstitial Pneumonia
- Child with Urinary Tract Infection
- Cytologic Findings of Bronchoalveolar Lavage