J Liver Cancer.
2020 Sep;20(2):160-166. 10.17998/jlc.20.2.160.
Advanced Stage Hepatocellular Carcinoma Successfully Treated with Transarterial Radioembolization and Multi-tyrosine Kinase Inhibitor Therapy
- Affiliations
-
- 1Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2508028
- DOI: http://doi.org/10.17998/jlc.20.2.160
Abstract
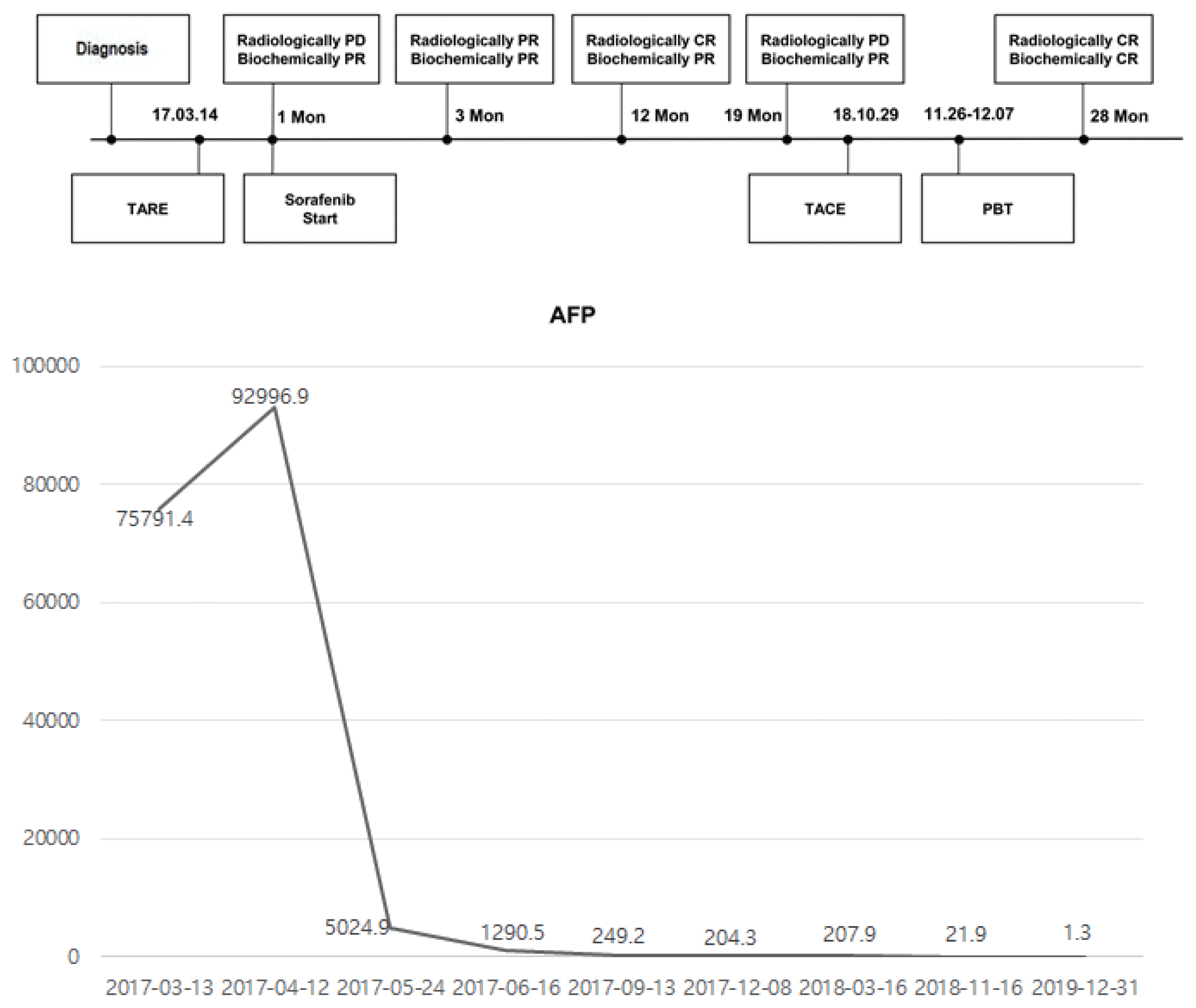
- Transarterial radioembolization (TARE) with yttrium-90 microspheres has become widely utilized in managing hepatocellular carcinoma (HCC). The utility of TARE is expanding with new insights through experiences from real-world practice and clinical trials, and recently published data suggest that TARE in combination with sorafenib may improve the overall survival in selected patients. Here, we report a case of advanced stage HCC that was successfully treated with TARE and sorafenib. The patient achieved complete response (CR) at 12 months after the initial treatment with TARE and sorafenib, followed by additional transarterial chemoembolization and proton beam therapy for local tumor recurrence at 19-month post-TARE. The patient was followed up every 3 months thereafter and still achieved CR both biochemically and radiologically for the following 12 months. A combination strategy of TARE and systemic therapy may be a useful alternative treatment option for selected patients with advanced stage HCC.
Keyword
Figure
Reference
-
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.2. Rawla P, Sunkara T, Muralidharan P, Raj JP. Update in global trends and aetiology of hepatocellular carcinoma. Contemp Oncol (Pozn). 2018; 22:141–150.3. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018; 67:358–380.4. Salem R, Gabr A, Riaz A, Mora R, Ali R, Abecassis M, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology. 2018; 68:1429–1440.5. Kim DY, Han KH. Transarterial chemoembolization versus transarterial radioembolization in hepatocellular carcinoma: optimization of selecting treatment modality. Hepatol Int. 2016; 10:883–892.6. Sangro B, Salem R. Transarterial chemoembolization and radioembolization. Semin Liver Dis. 2014; 34:435–443.7. Moreno-Luna LE, Yang JD, Sanchez W, Paz-Fumagalli R, Harnois DM, Mettler TA, et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013; 36:714–723.8. Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol. 2017; 67:999–1008.9. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34.10. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.11. Plastaras JP, Kim SH, Liu YY, Dicker DT, Dorsey JF, McDonough J, et al. Cell cycle dependent and schedule-dependent antitumor effects of sorafenib combined with radiation. Cancer Res. 2007; 67:9443–9454.12. Pracht M, Edeline J, Lepareur N, Lenoir L, Ardisson V, Clement B, et al. In vitro demonstration of synergy/additivity between (188) rhenium and sorafenib on hepatoma lines: preliminary results. Anticancer Res. 2013; 33:3871–3877.13. Ricke J, Bulla K, Kolligs F, Peck-Radosavljevic M, Reimer P, Sangro B, et al. Safety and toxicity of radioembolization plus Sorafenib in advanced hepatocellular carcinoma: analysis of the European multicentre trial SORAMIC. Liver Int. 2015; 35:620–626.14. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Korean J Radiol. 2019; 20:1042–1113.15. European Association for the Study of the Liver, Clinical practice guidelines panel, Wendon J, Panel M, Cordoba J, Dhawan A, et al. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017; 66:1047–1081.16. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.17. Kudo M, Arizumi T. Transarterial chemoembolization in combination with a Molecular Targeted Agent: Lessons Learned from Negative Trials (Post-TACE, BRISK-TA, SPACE, ORIENTAL, and TACE-2). Oncology. 2017; 93(Suppl 1):127–134.18. Forner A, Da Fonseca LG, Díaz-González Á, Sanduzzi-Zamparelli M, Reig M, Bruix J. Controversies in the management of hepatocellular carcinoma. JHEP Reps. 2019; 1:17–29.19. Ricke J, Klümpen HJ, Amthauer H, Bargellini I, Bartenstein P, de Toni EN, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol. 2019; 71:1164–1174.20. Chauhan N, Bukovcan J, Boucher E, Cosgrove D, Edeline J, Hamilton B, et al. Intra-Arterial TheraSphere Yttrium-90 Glass Microspheres in the treatment of patients with unresectable hepatocellular carcinoma: protocol for the STOP-HCC Phase 3 randomized controlled trial. JMIR Res Protoc. 2018; 7:e11234.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the therapeutic effects of transarterial radioembolization and tyrosine kinase inhibitor in hepatocellular carcinoma with portal vein thrombosis
- Reappraisal of transarterial radioembolization for liver-confined hepatocellular carcinoma with portal vein tumor thrombosis: Editorial on “Transarterial radioembolization versus tyrosine kinase inhibitor in hepatocellular carcinoma with portal vein thrombosis”
- Correspondence to letter to the editor on “Transarterial radioembolization versus tyrosine kinase inhibitor in hepatocellular carcinoma with portal vein thrombosis”
- Complications Related to Transarterial Treatment of Hepatocellular Carcinoma: A Comprehensive Review
- Nivolumab for Advanced Hepatocellular Carcinoma with Multiple Lung Metastases after Sorafenib Failure