Endocrinol Metab.
2020 Sep;35(3):628-635. 10.3803/EnM.2020.707.
Stimulated Salivary Cortisol as a Noninvasive Diagnostic Tool for Adrenal Insufficiency
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Mediplex Sejong Hospital, Incheon, Korea
- 3Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, Korea
- KMID: 2508013
- DOI: http://doi.org/10.3803/EnM.2020.707
Abstract
- Background
Salivary cortisol is routinely used as a diagnostic test for Cushing syndrome. The diagnostic use of salivary cortisol for adrenal insufficiency (AI), however, is less established. We aimed to investigate the utility of morning basal and adrenocorticotropic hormone-stimulated salivary cortisol in diagnosing AI in Korean adults.
Methods
We prospectively included 120 subjects (female, n=70) from Seoul National University Hospital. AI was defined as a stimulated serum cortisol level of <496.8 nmol/L during the short Synacthen test (SST). Serum and saliva samples were drawn between 8:00 AM and 10:00 AM. Salivary cortisol levels were measured using an enzyme immunoassay kit.
Results
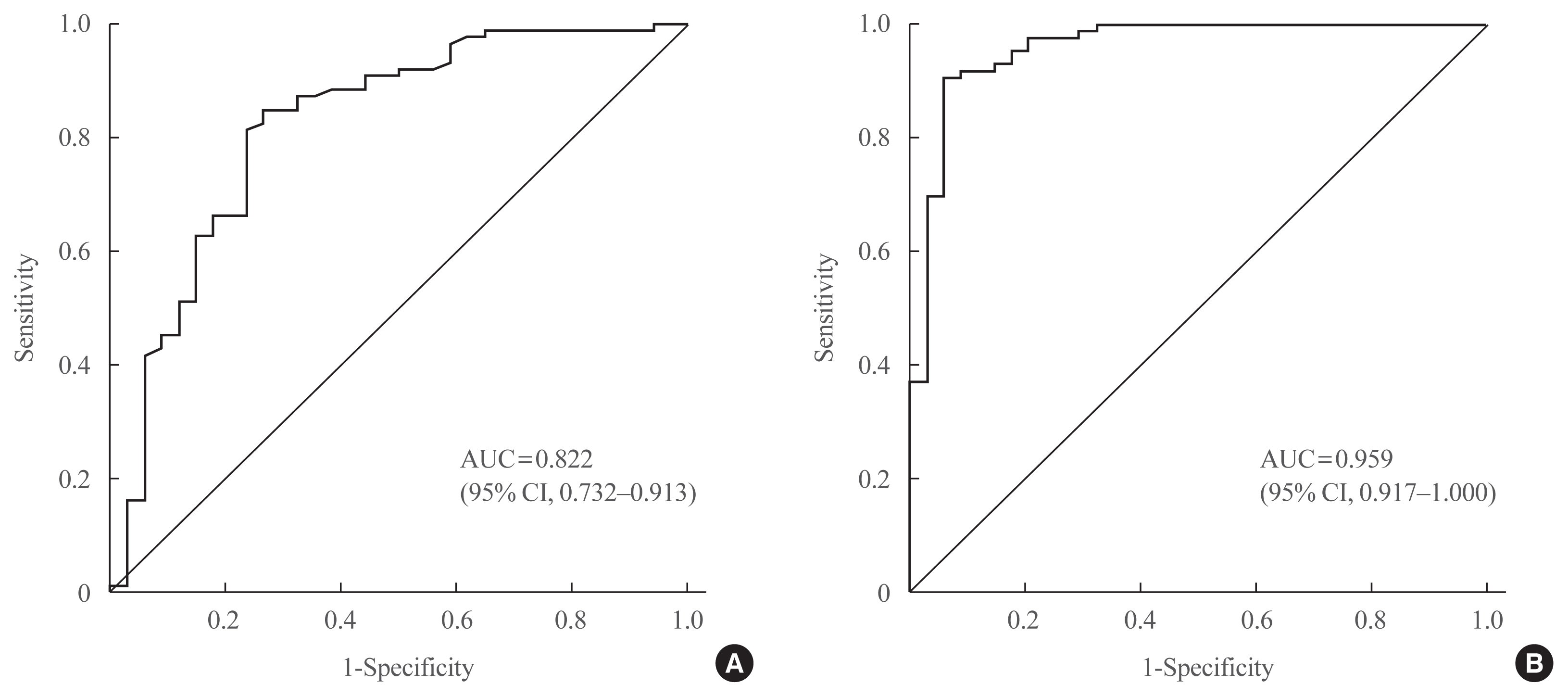
Thirty-four patients were diagnosed with AI according to the SST results. Age, sex, body mass index, serum albumin levels, and serum creatinine levels did not significantly differ between the normal and AI groups. Basal and stimulated salivary cortisol levels were positively correlated with basal (r=0.538) and stimulated serum cortisol levels (r=0.750), respectively (all P<0.001). Receiver operating characteristic curve analysis yielded a cutoff level of morning basal salivary cortisol of 3.2 nmol/L (sensitivity, 84.9%; specificity, 73.5%; area under the curve [AUC]=0.822). The optimal cutoff value of stimulated salivary cortisol was 13.2 nmol/L (sensitivity, 90.7%; specificity, 94.1%; AUC=0.959). Subjects with a stimulated salivary cortisol level above 13.2 nmol/L but a stimulated serum cortisol level below 496.8 nmol/L (n=2) had lower serum albumin levels than those showing a concordant response.
Conclusion
The diagnostic performance of stimulated salivary cortisol measurements after the SST was comparable to serum cortisol measurements for diagnosing AI.
Keyword
Figure
Cited by 1 articles
-
Clinical and Technical Aspects in Free Cortisol Measurement
Man Ho Choi
Endocrinol Metab. 2022;37(4):599-607. doi: 10.3803/EnM.2022.1549.
Reference
-
1. Stewart PM, Corrie J, Seckl JR, Edwards CR, Padfield PL. A rational approach for assessing the hypothalamo-pituitary-adrenal axis. Lancet. 1988; 1:1208–10.
Article2. Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003; 139:194–204.
Article3. Brien TG. Human corticosteroid binding globulin. Clin Endocrinol (Oxf). 1981; 14:193–212.
Article4. Coolens JL, Van Baelen H, Heyns W. Clinical use of unbound plasma cortisol as calculated from total cortisol and corticosteroid-binding globulin. J Steroid Biochem. 1987; 26:197–202.
Article5. Perogamvros I, Ray DW, Trainer PJ. Regulation of cortisol bioavailability: effects on hormone measurement and action. Nat Rev Endocrinol. 2012; 8:717–27.
Article6. Hammond GL. Determinants of steroid hormone bioavailability. Biochem Soc Trans. 1997; 25:577–82.
Article7. Arafah BM, Nishiyama FJ, Tlaygeh H, Hejal R. Measurement of salivary cortisol concentration in the assessment of adrenal function in critically ill subjects: a surrogate marker of the circulating free cortisol. J Clin Endocrinol Metab. 2007; 92:2965–71.
Article8. Wood P. Salivary steroid assays: research or routine? Ann Clin Biochem. 2009; 46(Pt 3):183–96.9. Groschl M. Current status of salivary hormone analysis. Clin Chem. 2008; 54:1759–69.
Article10. Dorn LD, Lucke JF, Loucks TL, Berga SL. Salivary cortisol reflects serum cortisol: analysis of circadian profiles. Ann Clin Biochem. 2007; 44(Pt 3):281–4.
Article11. Galbois A, Rudler M, Massard J, Fulla Y, Bennani A, Bonnefont-Rousselot D, et al. Assessment of adrenal function in cirrhotic patients: salivary cortisol should be preferred. J Hepatol. 2010; 52:839–45.
Article12. Inder WJ, Dimeski G, Russell A. Measurement of salivary cortisol in 2012: laboratory techniques and clinical indications. Clin Endocrinol (Oxf). 2012; 77:645–51.13. Riad-Fahmy D, Read GF, Walker RF. Salivary steroid assays for screening endocrine function. Postgrad Med J. 1980; 56(Suppl 1):75–8.14. Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008; 93:1526–40.
Article15. Ceccato F, Barbot M, Zilio M, Ferasin S, Occhi G, Daniele A, et al. Performance of salivary cortisol in the diagnosis of Cushing’s syndrome, adrenal incidentaloma, and adrenal insufficiency. Eur J Endocrinol. 2013; 169:31–6.
Article16. Langelaan MLP, Kisters JMH, Oosterwerff MM, Boer AK. Salivary cortisol in the diagnosis of adrenal insufficiency: cost efficient and patient friendly. Endocr Connect. 2018; 7:560–6.
Article17. Deutschbein T, Broecker-Preuss M, Flitsch J, Jaeger A, Althoff R, Walz MK, et al. Salivary cortisol as a diagnostic tool for Cushing’s syndrome and adrenal insufficiency: improved screening by an automatic immunoassay. Eur J Endocrinol. 2012; 166:613–8.
Article18. Restituto P, Galofre JC, Gil MJ, Mugueta C, Santos S, Monreal JI, et al. Advantage of salivary cortisol measurements in the diagnosis of glucocorticoid related disorders. Clin Biochem. 2008; 41:688–92.
Article19. Deutschbein T, Unger N, Mann K, Petersenn S. Diagnosis of secondary adrenal insufficiency in patients with hypothalamic-pituitary disease: comparison between serum and salivary cortisol during the high-dose short synacthen test. Eur J Endocrinol. 2009; 160:9–16.
Article20. Karpman MS, Neculau M, Dias VC, Kline GA. Defining adrenal status with salivary cortisol by gold-standard insulin hypoglycemia. Clin Biochem. 2013; 46:1442–6.
Article21. Contreras LN, Arregger AL, Persi GG, Gonzalez NS, Cardoso EM. A new less-invasive and more informative low-dose ACTH test: salivary steroids in response to intramuscular corticotrophin. Clin Endocrinol (Oxf). 2004; 61:675–82.
Article22. Laudat MH, Cerdas S, Fournier C, Guiban D, Guilhaume B, Luton JP. Salivary cortisol measurement: a practical approach to assess pituitary-adrenal function. J Clin Endocrinol Metab. 1988; 66:343–8.
Article23. Cornes MP, Ashby HL, Khalid Y, Buch HN, Ford C, Gama R. Salivary cortisol and cortisone responses to tetracosactrin (synacthen). Ann Clin Biochem. 2015; 52(Pt 5):606–10.
Article24. Raff H, Homar PJ, Skoner DP. New enzyme immunoassay for salivary cortisol. Clin Chem. 2003; 49:203–4.
Article25. Kosak M, Hana V, Hill M, Simunkova K, Lacinova Z, Krsek M, et al. Serum cortisol seems to be a more appropriate marker for adrenocortical reserve evaluation in ACTH test in comparison to salivary cortisol. Physiol Res. 2014; 63:229–36.
Article26. Simunkova K, Hampl R, Hill M, Doucha J, Starka L, Vondra K. Salivary cortisol in low dose (1 microg) ACTH test in healthy women: comparison with serum cortisol. Physiol Res. 2007; 56:449–53.27. Marcus-Perlman Y, Tordjman K, Greenman Y, Limor R, Shenkerman G, Osher E, et al. Low-dose ACTH (1 microg) salivary test: a potential alternative to the classical blood test. Clin Endocrinol (Oxf). 2006; 64:215–8.28. Nolan BJ, Sorbello J, Brown N, Dimeski G, Inder WJ. Characterization of the serum and salivary cortisol response to the intravenous 250 μg ACTH 1–24 stimulation test. Endocrine. 2018; 59:520–8.
Article29. Dickstein G, Shechner C, Nicholson WE, Rosner I, Shen-Orr Z, Adawi F, et al. Adrenocorticotropin stimulation test: effects of basal cortisol level, time of day, and suggested new sensitive low dose test. J Clin Endocrinol Metab. 1991; 72:773–8.
Article30. Cho HY, Kim JH, Kim SW, Shin CS, Park KS, Kim SW, et al. Different cut-off values of the insulin tolerance test, the high-dose short Synacthen test (250 μg) and the low-dose short Synacthen test (1 μg) in assessing central adrenal insufficiency. Clin Endocrinol (Oxf). 2014; 81:77–84.
Article31. Elder CJ, Harrison RF, Cross AS, Vilela R, Keevil BG, Wright NP, et al. Use of salivary cortisol and cortisone in the high- and low-dose synacthen test. Clin Endocrinol (Oxf). 2018; 88:772–8.
Article32. Arafah BM. Hypothalamic pituitary adrenal function during critical illness: limitations of current assessment methods. J Clin Endocrinol Metab. 2006; 91:3725–45.
Article33. Meuwese CL, Carrero JJ. Chronic kidney disease and hypothalamic-pituitary axis dysfunction: the chicken or the egg? Arch Med Res. 2013; 44:591–600.
Article34. Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004; 350:1629–38.
Article35. Arregger AL, Cardoso EM, Zucchini A, Aguirre EC, Elbert A, Contreras LN. Adrenocortical function in hypotensive patients with end stage renal disease. Steroids. 2014; 84:57–63.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of salivary and serum cortisol levels in mechanically ventilated patients and non-critically ill patients
- Evaluation of function and disorders of the adrenal gland in neonates
- Usefullness of Urinary Free Cortisol Measurement in Diagnosis of Iatrogenic Cushing Syndrome
- Urine Free Cortisol and Secondary Adrenal Insufficiency
- The Maternal and Fetal Adrenal Effect of 1 cycle Dexamethasone on women with Preterm Labor