J Korean Med Sci.
2020 Oct;35(41):e374. 10.3346/jkms.2020.35.e374.
Bladder Regeneration Using a Polycaprolactone Scaffold with a Gradient Structure and Growth Factors in a Partially Cystectomized Rat Model
- Affiliations
-
- 1Department of Nanobiomedical Science & BK21 PLUS NBM Global Research Center for Regenerative Medicine, Dankook University, Cheonan, Korea
- 2BioMedical Research Institute, Kyungpook National University Hospital, Daegu, Korea
- 3Joint Institution for Regenerative Medicine, Kyungpook National University Hospital, Daegu, Korea
- 4Department of Urology, Kyungpook National University Hospital, Daegu, Korea
- 5Department of Urology, School of Medicine, Kyungpook National University, Daegu, Korea
- 6Department of Urology, Kyungpook National University Chilgok Hospital, Daegu, Korea
- KMID: 2507829
- DOI: http://doi.org/10.3346/jkms.2020.35.e374
Abstract
- Background
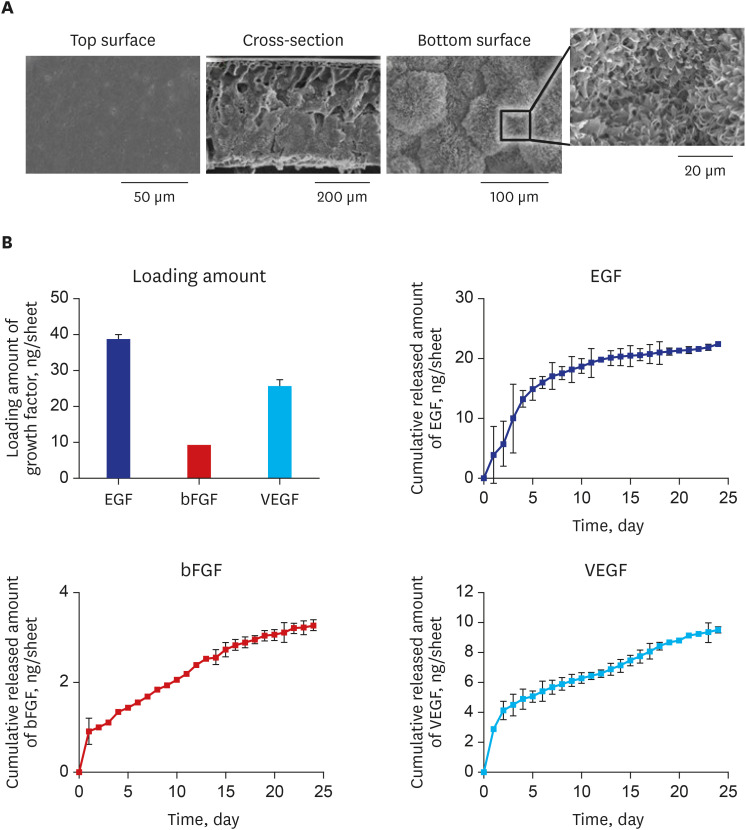
Tissue engineering can be used for bladder augmentation. However, conventional scaffolds result in fibrosis and graft shrinkage. This study applied an alternative polycaprolactone (PCL)-based scaffold (diameter = 5 mm) with a noble gradient structure and growth factors (GFs) (epidermal growth factor, vascular endothelial growth factor, and basic fibroblast growth factor) to enhance bladder tissue regeneration in a rat model.
Methods
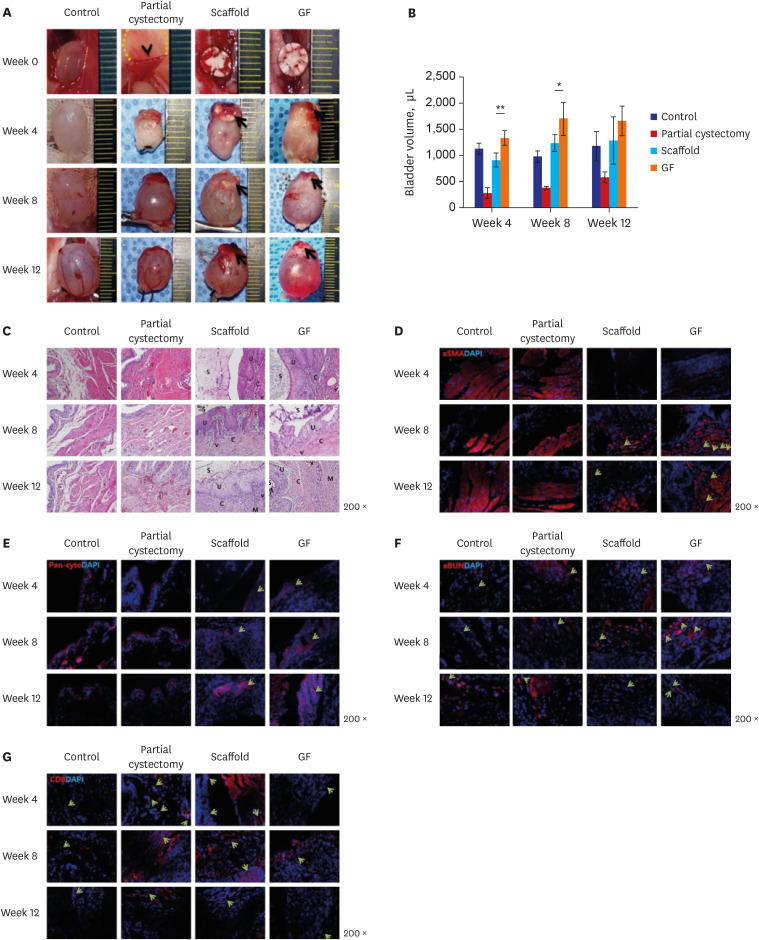
Partially excised urinary bladders of 5-week-old male Slc:SD rats were reconstructed with the scaffold (scaffold group) or the scaffold combined with GFs (GF group) and compared with sham-operated (control group) and untreated rats (partial cystectomy group). Evaluations of bladder volume, histology, immunohistochemistry (IHC), and molecular markers were performed at 4, 8, and 12 weeks after operation.
Results
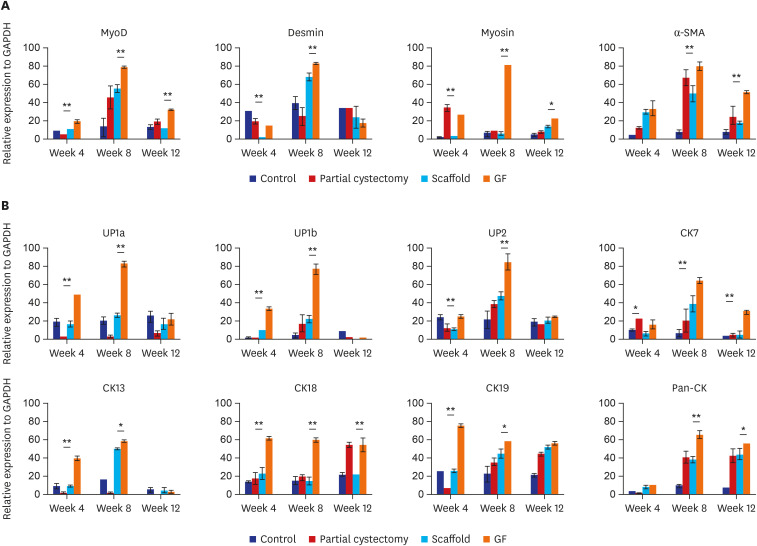
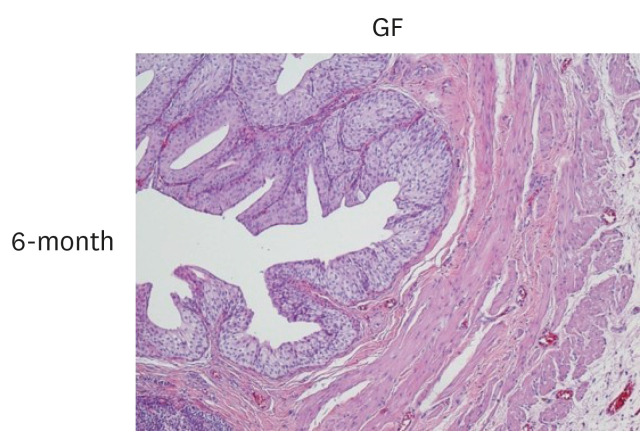
The bladder volumes of the scaffold and GF group recovered to the normal range, and those of the GF group showed more enhanced augmentation. Histological evaluations revealed that the GF group showed more organized urothelial lining, dense extracellular matrix, frequent angiogenesis, and enhanced smooth muscle bundle regeneration than the scaffold group. IHC for α-smooth muscle actin, pan-cytokeratin, α-bungarotoxin, and CD8 revealed that the GF group showed high formation of smooth muscle, blood vessel, urothelium, neuromuscular junction and low immunogenicity. Concordantly, real-time polymerase chain reaction experiments revealed that the GF group showed a higher expression of transcripts associated with smooth muscle and urothelial differentiation. In a 6-month in vivo safety analysis, the GF group showed normal histology.
Conclusion
This study showed that a PCL scaffold with a gradient structure incorporating GFs improved bladder regeneration functionally and histologically.
Figure
Reference
-
1. Mills RD, Studer UE. Metabolic consequences of continent urinary diversion. J Urol. 1999; 161(4):1057–1066. PMID: 10081838.
Article2. Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006; 367(9518):1241–1246. PMID: 16631879.
Article3. Zhao Y, He Y, Guo JH, Wu JS, Zhou Z, Zhang M, et al. Time-dependent bladder tissue regeneration using bilayer bladder acellular matrix graft-silk fibroin scaffolds in a rat bladder augmentation model. Acta Biomater. 2015; 23:91–102. PMID: 26049152.
Article4. Lee JN, Chun SY, Lee HJ, Jang YJ, Choi SH, Kim DH, et al. Human urine-derived stem cells seeded surface modified composite scaffold grafts for bladder reconstruction in a rat model. J Korean Med Sci. 2015; 30(12):1754–1763. PMID: 26713050.
Article5. Di Luca A, Szlazak K, Lorenzo-Moldero I, Ghebes CA, Lepedda A, Swieszkowski W, et al. Influencing chondrogenic differentiation of human mesenchymal stromal cells in scaffolds displaying a structural gradient in pore size. Acta Biomater. 2016; 36:210–219. PMID: 26969523.
Article6. Nillesen ST, Geutjes PJ, Wismans R, Schalkwijk J, Daamen WF, van Kuppevelt TH. Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials. 2007; 28(6):1123–1131. PMID: 17113636.
Article7. Lee SH, Jin WP, Seo NR, Pang KM, Kim B, Kim SM, et al. Recombinant human fibroblast growth factor-2 promotes nerve regeneration and functional recovery after mental nerve crush injury. Neural Regen Res. 2017; 12(4):629–636. PMID: 28553345.
Article8. Freeman MR, Yoo JJ, Raab G, Soker S, Adam RM, Schneck FX, et al. Heparin-binding EGF-like growth factor is an autocrine growth factor for human urothelial cells and is synthesized by epithelial and smooth muscle cells in the human bladder. J Clin Invest. 1997; 99(5):1028–1036. PMID: 9062361.
Article9. Roelofs LA, Kortmann BB, Oosterwijk E, Eggink AJ, Tiemessen DM, Crevels AJ, et al. Tissue engineering of diseased bladder using a collagen scaffold in a bladder exstrophy model. BJU Int. 2014; 114(3):447–457. PMID: 25302355.
Article10. Kanematsu A, Yamamoto S, Noguchi T, Ozeki M, Tabata Y, Ogawa O. Bladder regeneration by bladder acellular matrix combined with sustained release of exogenous growth factor. J Urol. 2003; 170(4 Pt 2):1633–1638. PMID: 14501679.
Article11. Di Luca A, Ostrowska B, Lorenzo-Moldero I, Lepedda A, Swieszkowski W, Van Blitterswijk C, et al. Gradients in pore size enhance the osteogenic differentiation of human mesenchymal stromal cells in three-dimensional scaffolds. Sci Rep. 2016; 6:22898. PMID: 26961859.
Article12. Kellomäki M, Niiranen H, Puumanen K, Ashammakhi N, Waris T, Törmälä P. Bioabsorbable scaffolds for guided bone regeneration and generation. Biomaterials. 2000; 21(24):2495–2505. PMID: 11071599.
Article13. Guarino V, Ambrosio L. Electrofluidodynamic Technologies (EFDTs) for Biomaterials and Medical Devices: Principles and Advances. Duxford: Woodhead Publishing;2018.14. Deng Y, Kuiper J. Functional 3D Tissue Engineering Scaffolds: Materials, Technologies, and Applications. Duxford: Woodhead Publishing;2017.15. Chen W, Shi C, Hou X, Zhang W, Li L. Bladder acellular matrix conjugated with basic fibroblast growth factor for bladder regeneration. Tissue Eng Part A. 2014; 20(15-16):2234–2242. PMID: 24483233.
Article16. Khang G. Handbook of Intelligent Scaffolds for Tissue Engineering and Regenerative Medicine. Singapore: Pan Stanford Publishing;2017.17. Wefer J, Sievert KD, Schlote N, Wefer AE, Nunes L, Dahiya R, et al. Time dependent smooth muscle regeneration and maturation in a bladder acellular matrix graft: histological studies and in vivo functional evaluation. J Urol. 2001; 165(5):1755–1759. PMID: 11342970.
Article18. Beqaj SH, Donovan JL, Liu DB, Harrington DA, Alpert SA, Cheng EY. Role of basic fibroblast growth factor in the neuropathic bladder phenotype. J Urol. 2005; 174(4 Pt 2):1699–1703. PMID: 16148685.
Article19. Imamura M, Kanematsu A, Yamamoto S, Kimura Y, Kanatani I, Ito N, et al. Basic fibroblast growth factor modulates proliferation and collagen expression in urinary bladder smooth muscle cells. Am J Physiol Renal Physiol. 2007; 293(4):F1007–17. PMID: 17634401.
Article20. Borer JG, Park JM, Atala A, Nguyen HT, Adam RM, Retik AB, et al. Heparin-binding EGF-like growth factor expression increases selectively in bladder smooth muscle in response to lower urinary tract obstruction. Lab Invest. 1999; 79(11):1335–1345. PMID: 10576204.21. Young HS, Herbette LG, Skita V. α-bungarotoxin binding to acetylcholine receptor membranes studied by low angle X-ray diffraction. Biophys J. 2003; 85(2):943–953. PMID: 12885641.
Article22. Zheng C, Sköld MK, Li J, Nennesmo I, Fadeel B, Henter JI. VEGF reduces astrogliosis and preserves neuromuscular junctions in ALS transgenic mice. Biochem Biophys Res Commun. 2007; 363(4):989–993. PMID: 17923114.
Article23. Chen C. Presynaptic Differentiation at the Neuromuscular Junction: Regulation by a Novel bFGF-p120 Catenin Signaling Pathway [dissertation]. Hong Kong: Hong Kong University of Science and Technology;2007.24. Vishwakarma A, Sharpe P, Shi S, Ramalingam M. Stem Cell Biology and Tissue Engineering in Dental Sciences. Amsterdam: Academic Press;2014.25. Goustin AS, Leof EB, Shipley GD, Moses HL. Growth factors and cancer. Cancer Res. 1986; 46(3):1015–1029. PMID: 3002607.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Breast Tissue Reconstruction Using Polycaprolactone Ball Scaffolds in a Partial Mastectomy Pig Model
- Human Urine-derived Stem Cells Seeded Surface Modified Composite Scaffold Grafts for Bladder Reconstruction in a Rat Model
- Changes of Dopaminergic Neurons in Parkinson's Rat Model after Fetal Striatal Transplantation
- Hybrid Additive Microfabrication Scaffold Incorporated with Highly Aligned Nanofibers for Musculoskeletal Tissues
- Assessment of Bacterial Communities Within the Biofilm of Bladder Calculi in the Neurogenic Bladder Rat Model Following Spinal Cord Injury