J Korean Med Sci.
2020 Oct;35(41):e342. 10.3346/jkms.2020.35.e342.
Strategy for Prostate Cancer Patients with Low Prostate Specific Antigen Level (2.5 to 4.0 ng/mL)
- Affiliations
-
- 1Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2507825
- DOI: http://doi.org/10.3346/jkms.2020.35.e342
Abstract
- Background
To evaluate the strategy for detection of prostate cancer (PCa) with low prostate specific antigen (PSA) level (2.5–4.0 ng/mL), prostate biopsy patients with low PSA were assessed. We evaluated the risk of low PSA PCa and the strategy for screening low-PSA patients.
Methods
We retrospectively analyzed the patients who underwent prostate biopsy with low PSA level. Baseline characteristics, PSA level before prostate biopsy, prostate volume, prostate specific antigen density (PSAD), and pathological data were assessed.
Results
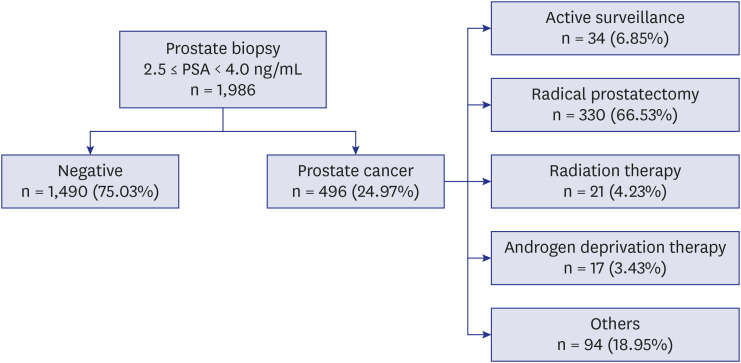
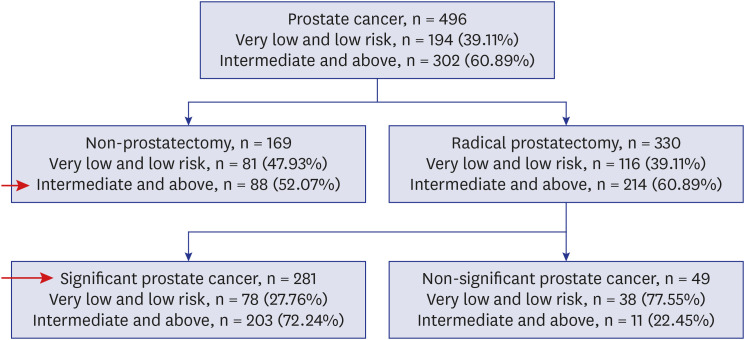
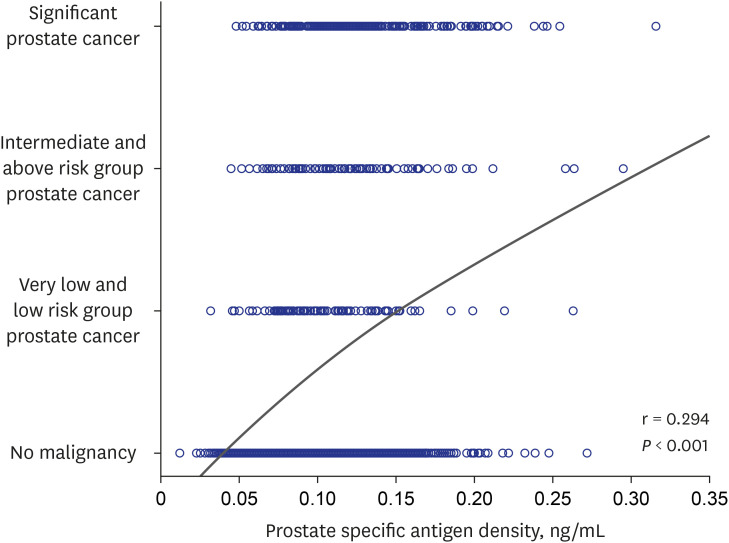
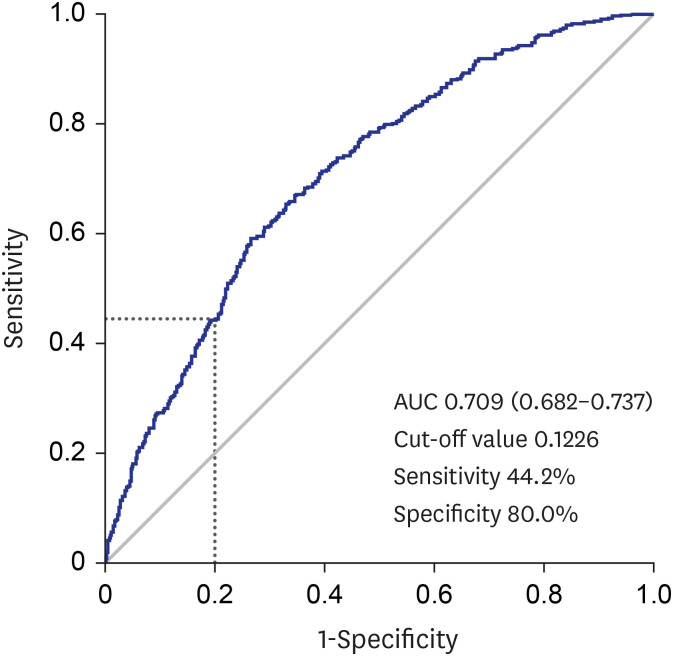
Among the 1986 patients, 24.97% were diagnosed with PCa. The PSAD was 0.12 ± 0.04 ng/mL2 in the PCa-diagnosed group and 0.10 ± 0.04 ng/mL2 in non-cancer-diagnosed group (P < 0.001). Of the 496 patients diagnosed with PCa, 302 (60.89%) were in the intermediate- or high-risk group. PSAD was 0.13 ± 0.04 ng/mL2 in the intermediate- or highrisk group and 0.11 ± 0.03 ng/mL2 in the very low- and low-risk group (P < 0.001). Of 330 patients who underwent radical prostatectomy, 85.15% were diagnosed as having significant cancer. There was significant correlation between PSAD and PCa (r = 0.294, P < 0.001). PSAD with a specificity of 80.00% of a clinically significant cancer diagnosis was assessed at 0.1226 ng/mL2 .
Conclusion
The PCa detection rate in the low-PSA group was not lower than that of previous studies of patients with PSA from 4.0 to 10.0 ng/mL. Further, it may be helpful to define a strategy for PCa detection using PSAD in the low-PSA group.
Figure
Reference
-
1. Verma A, St Onge J, Dhillon K, Chorneyko A. PSA density improves prediction of prostate cancer. Can J Urol. 2014; 21(3):7312–7321. PMID: 24978363.2. Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012; 104(2):125–132. PMID: 22228146.3. Littrup PJ, Kane RA, Mettlin CJ, Murphy GP, Lee F, Toi A, et al. Cost-effective prostate cancer detection. Reduction of low-yield biopsies. Cancer. 1994; 74(12):3146–3158. PMID: 7526969.
Article4. Catalona WJ, Smith DS, Ornstein DK. Prostate cancer detection in men with serum PSA concentrations of 2.6 to 4.0 ng/mL and benign prostate examination. Enhancement of specificity with free PSA measurements. JAMA. 1997; 277(18):1452–1455. PMID: 9145717.
Article5. Schröder FH, van der Cruijsen-Koeter I, de Koning HJ, Vis AN, Hoedemaeker RF, Kranse R. Prostate cancer detection at low prostate specific antigen. J Urol. 2000; 163(3):806–812. PMID: 10687982.6. Kim HS, Jeon SS, Choi JD, Kim W, Han DH, Jeong BC, et al. Detection rates of nonpalpable prostate cancer in Korean men with prostate-specific antigen levels between 2.5 and 4.0 ng/mL. Urology. 2010; 76(4):919–922. PMID: 20303152.
Article7. Babaian RJ, Fritsche H, Ayala A, Bhadkamkar V, Johnston DA, Naccarato W, et al. Performance of a neural network in detecting prostate cancer in the prostate-specific antigen reflex range of 2.5 to 4.0 ng/mL. Urology. 2000; 56(6):1000–1006. PMID: 11113747.
Article8. Kobayashi T, Nishizawa K, Ogura K, Mitsumori K, Ide Y. Detection of prostate cancer in men with prostate-specific antigen levels of 2.0 to 4.0 ng/mL equivalent to that in men with 4.1 to 10.0 ng/mL in a Japanese population. Urology. 2004; 63(4):727–731. PMID: 15072889.
Article9. Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994; 271(5):368–374. PMID: 7506797.
Article10. Catalona WJ, Richie JP, Ahmann FR, Hudson MA, Scardino PT, Flanigan RC, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol. 1994; 151(5):1283–1290. PMID: 7512659.
Article11. Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJ, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991; 324(17):1156–1161. PMID: 1707140.
Article12. Thompson IM, Ankerst DP, Chi C, Goodman PJ, Tangen CM, Lucia MS, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006; 98(8):529–534. PMID: 16622122.
Article13. Togo Y, Yamamoto S. Prevention of infectious complications after prostate biopsy procedure. Int J Urol. 2017; 24(7):486–492. PMID: 28556409.
Article14. Fowler FJ Jr, Barry MJ, Walker-Corkery B, Caubet JF, Bates DW, Lee JM, et al. The impact of a suspicious prostate biopsy on patients' psychological, socio-behavioral, and medical care outcomes. J Gen Intern Med. 2006; 21(7):715–721. PMID: 16808772.
Article15. Kearns JT, Lin DW. Improving the specificity of PSA screening with serum and urine markers. Curr Urol Rep. 2018; 19(10):80. PMID: 30105509.
Article16. Christensson A, Björk T, Nilsson O, Dahlén U, Matikainen MT, Cockett AT, et al. Serum prostate specific antigen complexed to alpha 1-antichymotrypsin as an indicator of prostate cancer. J Urol. 1993; 150(1):100–105. PMID: 7685416.17. Catalona WJ, Partin AW, Sanda MG, Wei JT, Klee GG, Bangma CH, et al. A multicenter study of [-2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range. J Urol. 2011; 185(5):1650–1655. PMID: 21419439.
Article18. Vickers AJ, Cronin AM, Aus G, Pihl CG, Becker C, Pettersson K, et al. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: data from the European Randomized Study of Prostate Cancer Screening in Göteborg, Sweden. BMC Med. 2008; 6(1):19. PMID: 18611265.
Article19. Bussemakers MJ, van Bokhoven A, Verhaegh GW, Smit FP, Karthaus HF, Schalken JA, et al. DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 1999; 59(23):5975–5979. PMID: 10606244.20. Leyten GH, Hessels D, Smit FP, Jannink SA, de Jong H, Melchers WJ, et al. Identification of a candidate gene panel for the early diagnosis of prostate cancer. Clin Cancer Res. 2015; 21(13):3061–3070. PMID: 25788493.
Article21. Donovan MJ, Noerholm M, Bentink S, Belzer S, Skog J, O'Neill V, et al. A molecular signature of PCA3 and ERG exosomal RNA from non-DRE urine is predictive of initial prostate biopsy result. Prostate Cancer Prostatic Dis. 2015; 18(4):370–375. PMID: 26345389.
Article22. Fütterer JJ. Multiparametric MRI in the Detection of Clinically Significant Prostate Cancer. Korean J Radiol. 2017; 18(4):597–606. PMID: 28670154.
Article23. Esen T, Kilic M, Seymen H, Acar O, Demirkol MO. Can Ga-68 PSMA PET/CT replace conventional imaging modalities for primary lymph node and bone staging of prostate cancer? Eur Urol Focus. 2020; 6(2):218–220. PMID: 31113757.
Article24. Kundu SD, Roehl KA, Yu X, Antenor JA, Suarez BK, Catalona WJ. Prostate specific antigen density correlates with features of prostate cancer aggressiveness. J Urol. 2007; 177(2):505–509. PMID: 17222621.
Article25. Liu J, Wang ZQ, Li M, Zhou MY, Yu YF, Zhan WW. Establishment of two new predictive models for prostate cancer to determine whether to require prostate biopsy when the PSA level is in the diagnostic gray zone (4–10 ng ml-1). Asian J Androl. 2020; 22(2):213–216. PMID: 31169140.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prostate specific antigen as a tumor marker for adenocarcinoma of the prostate
- Association of Prostate Specific Antigen (PSA) Velocity with Age and Initial PSA in Healthy Korean Men
- Effects of Medication with Dutasteride on Detection of Prostate Cancer in Patients with Serum Prostate-specific Antigen Level of 4~10 ng/ml
- Predictive Factors for Prostate Cancer in Biopsy of Patients with Prostate-Specific Antigen Levels Equal to or Less Than 4 ng/ml
- Is it Appropriate to Lower the Prostate Specific Antigen Cut-off Value to 2.5 ng/ml for Prostate Biopsy in Korean?