Korean J Gastroenterol.
2020 Oct;76(4):206-210. 10.4166/kjg.2020.76.4.206.
Gastric Gastrointestinal Stromal Tumor with Repeated Recurrence at the Anastomosis Site in a Very Elderly Patient
- Affiliations
-
- 1Department of Internal Medicine, Pathology, Chung-Ang University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Surgery, Chung-Ang University College of Medicine, Seoul, Korea
- KMID: 2507768
- DOI: http://doi.org/10.4166/kjg.2020.76.4.206
Abstract
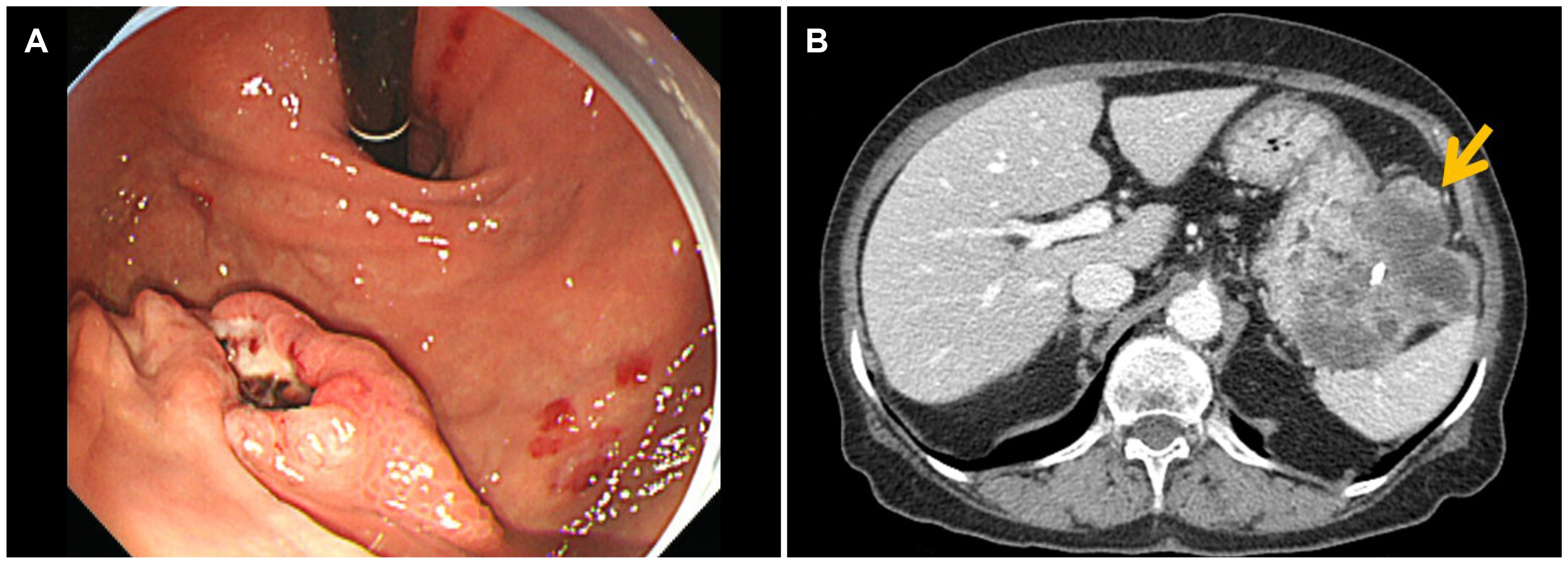
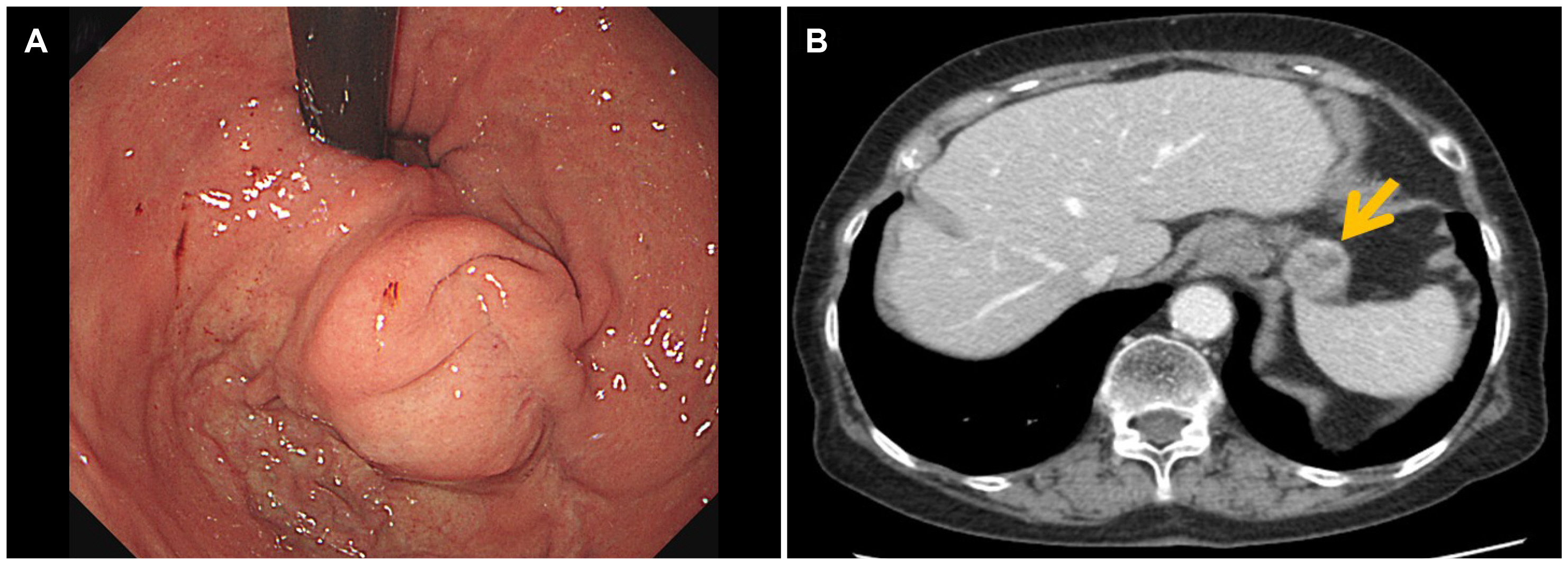
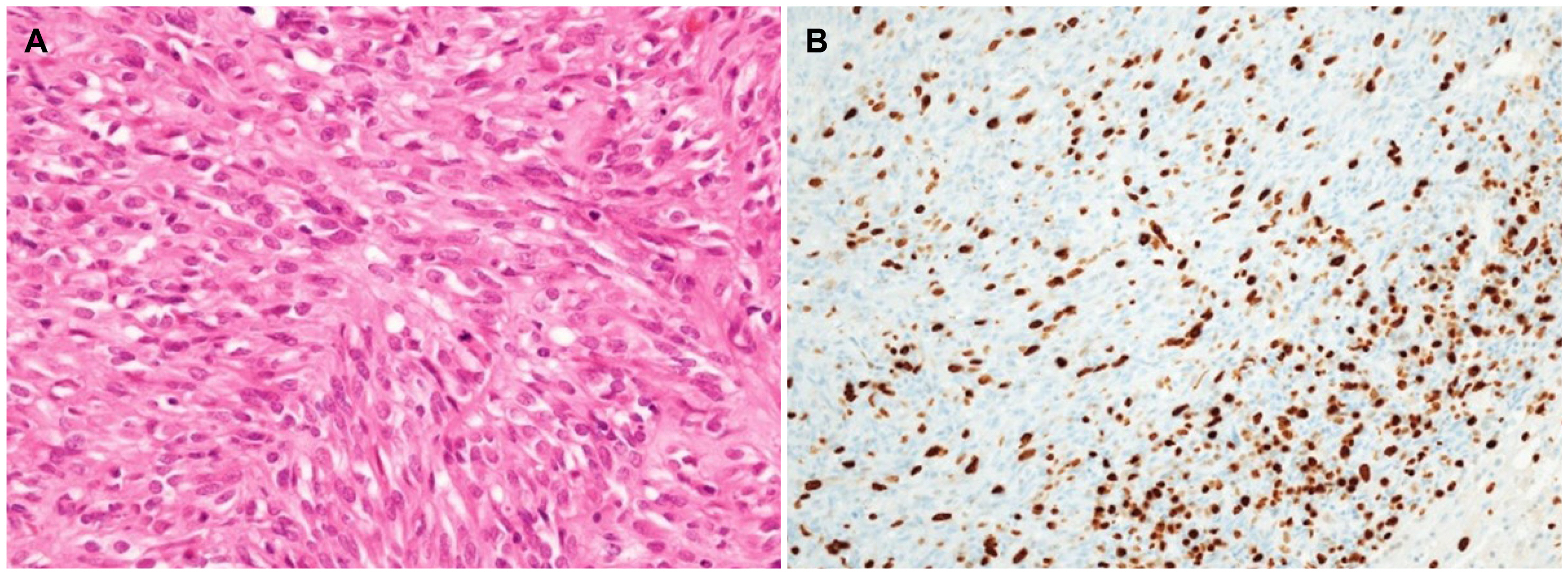
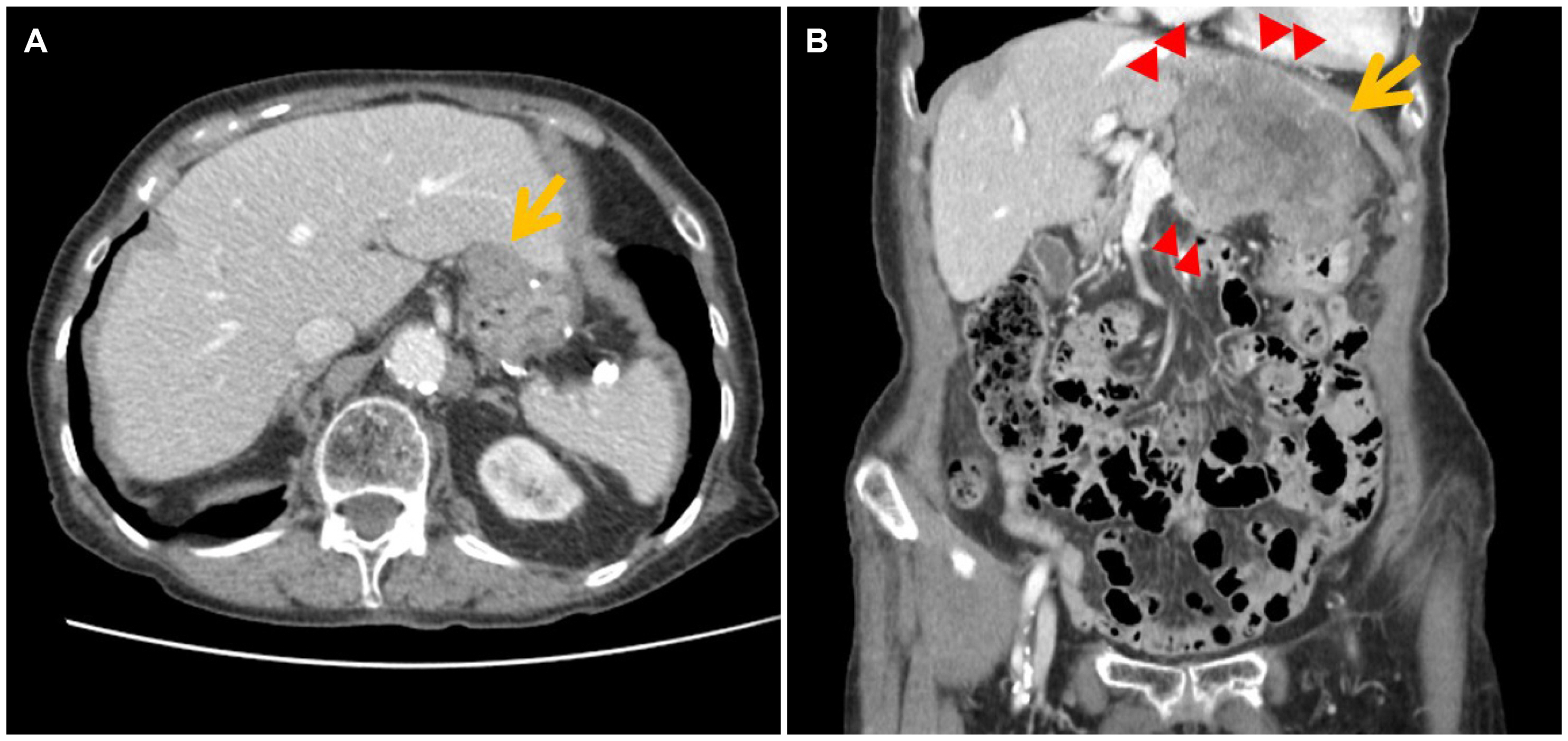
- Although high-risk gastrointestinal stromal tumors (GISTs) frequently recur, even after a complete resection and imatinib therapy, local recurrence at the suture line after complete resection is rare. The present case was an 88-year-old woman who was initially diagnosed with high-risk GIST without a distant metastasis. She underwent a complete surgical resection of the lesion and received adjuvant imatinib therapy for 18 months, which was discontinued due to severe drug-induced anemia. During the follow-up, an endoscopic examination performed 40 months after the initial surgery revealed local recurrence at the anastomosis site. Although a complete surgical resection was performed, repeated local recurrence was detected 18 months later, which progressed rapidly to metastatic disease. This paper reports a case of a completely resected gastric GIST with repeated local recurrence, despite the complete surgical resections and adjuvant imatinib therapy.
Figure
Reference
-
1. Sorour MA, Kassem MI, Ghazal Ael-H, El-Riwini MT, Abu Nasr A. 2014; Gastrointestinal stromal tumors (GIST) related emergencies. Int J Surg. 12:269–280. DOI: 10.1016/j.ijsu.2014.02.004. PMID: 24530605.
Article2. Deshaies I, Cherenfant J, Gusani NJ, et al. 2010; Gastrointestinal stromal tumor (GIST) recurrence following surgery: review of the clin-ical utility of imatinib treatment. Ther Clin Risk Manag. 6:453–458. DOI: 10.2147/TCRM.S5634. PMID: 20957137. PMCID: PMC2952484.3. Zhao R, Wang Y, Huang Y, et al. 2017; Adjuvant imatinib for patients with high-risk gastrointestinal stromal tumors: a retrospective cohort study. Sci Rep. 7:16834. DOI: 10.1038/s41598-017-17266-5. PMID: 29203825. PMCID: PMC5715066.
Article4. Raut CP, Espat NJ, Maki RG, et al. 2018; Efficacy and tolerability of 5-year adjuvant imatinib treatment for patients with resected intermediate- or high-risk primary gastrointestinal stromal tumor: the PERSIST-5 clinical trial. JAMA Oncol. 4:e184060. DOI: 10.1001/jamaoncol.2018.4060. PMID: 30383140. PMCID: PMC6440723.5. Joensuu H, Eriksson M, Sundby Hall K, et al. 2012; One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 307:1265–1272. DOI: 10.1001/jama.2012.347. PMID: 22453568.6. Judson I, Bulusu R, Seddon B, Dangoor A, Wong N, Mudan S. 2017; UK clinical practice guidelines for the management of gastrointestinal stromal tumours (GIST). Clin Sarcoma Res. 7:6. DOI: 10.1186/s13569-017-0072-8. PMID: 28465823. PMCID: PMC5408425.
Article7. Demetri GD, von Mehren M, Blanke CD, et al. 2002; Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 347:472–480. DOI: 10.1056/NEJMoa020461. PMID: 12181401.8. Blanke CD, Rankin C, Demetri GD, et al. 2008; Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 26:626–632. DOI: 10.1200/JCO.2007.13.4452. PMID: 18235122.
Article9. Latagliata R, Ferrero D, Iurlo A, et al. 2013; Imatinib in very elderly patients with chronic myeloid leukemia in chronic phase: a retrospective study. Drugs Aging. 30:629–637. DOI: 10.1007/s40266-013-0088-6. PMID: 23681399.
Article10. Ogata K, Kimura A, Nakazawa N, et al. 2018; Long-term imatinib treatment for patients with unresectable or recurrent gastrointestinal stromal tumors. Digestion. 97:20–25. DOI: 10.1159/000484102. PMID: 29393163.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Epithelioid Type Gastric Gastrointestinal Stromal Tumor with Gastrointestinal Bleeding
- Association between Recurrence and Survival Rates According to the Location of Gastric Gastrointestinal Stromal Tumor
- Systemic Treatment of the Gastrointestinal Stromal Tumor (GIST)
- Extragastrointestinal Stromal Tumor Mimicking Gastric Subepithelial Tumor
- Tumor Size is Associated with Long-term Outcomes after Resection of Gastric Gastrointestinal Stromal Tumors