Korean J Physiol Pharmacol.
2020 Nov;24(6):473-479. 10.4196/kjpp.2020.24.6.473.
2,3,5,4’-Tetrahydroxystilbene-2-O-β-D-Glucoside modulated human umbilical vein endothelial cells injury under oxidative stress
- Affiliations
-
- 1College of Basic Medicine & Public Health, Zhejiang Chinese Medical University, Hangzhou, Zhejiang 310053, China
- 2College of Life Science, Zhejiang Chinese Medical University, Hangzhou, Zhejiang 310053, China
- KMID: 2507730
- DOI: http://doi.org/10.4196/kjpp.2020.24.6.473
Abstract
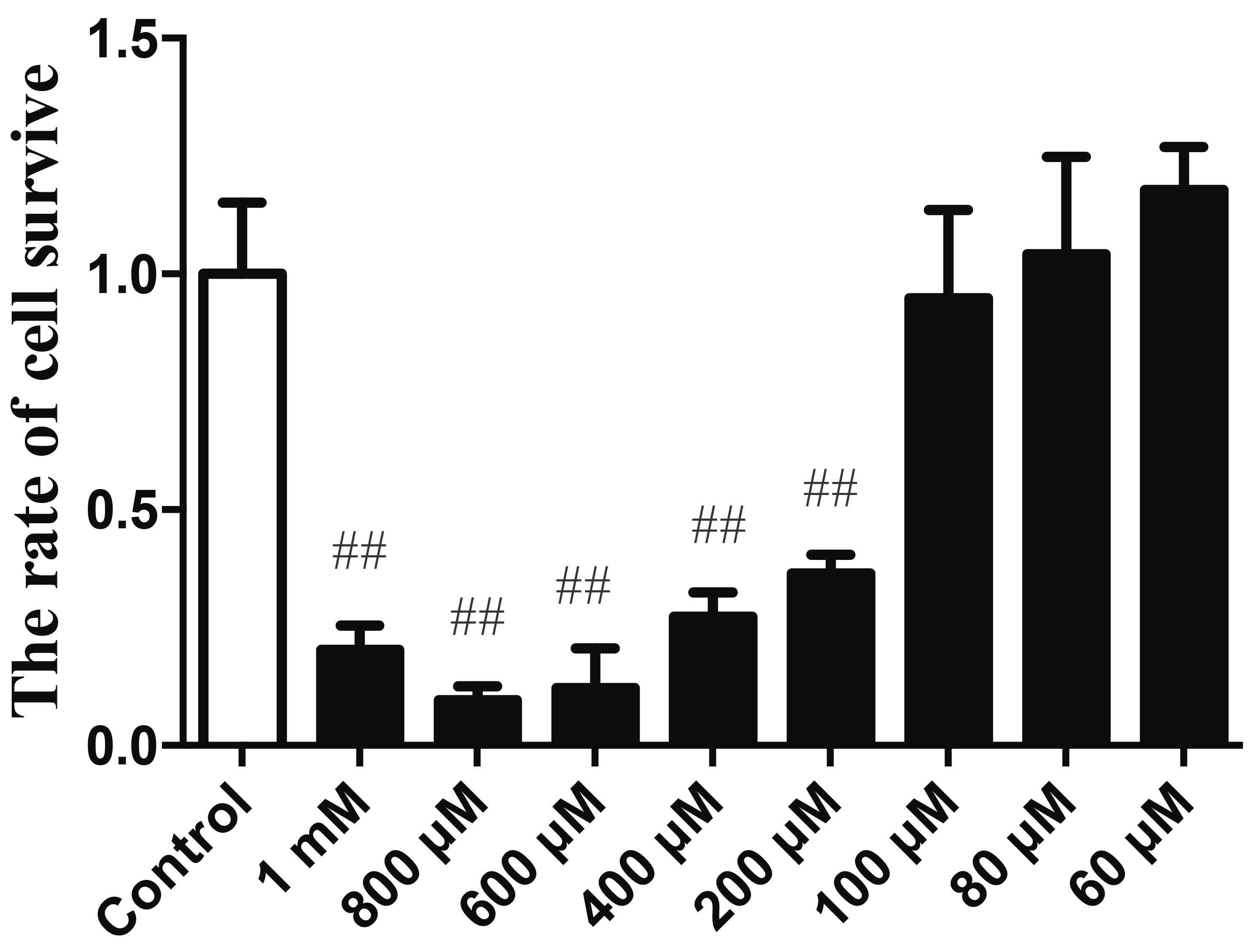
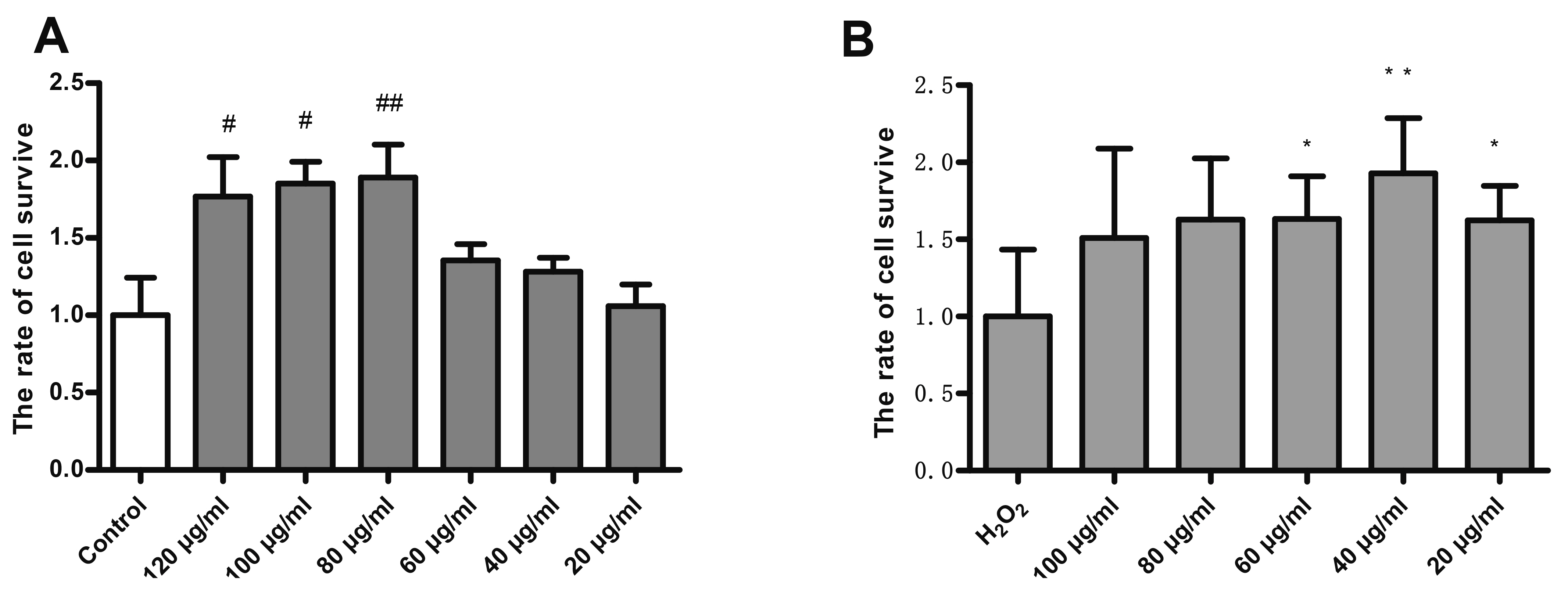
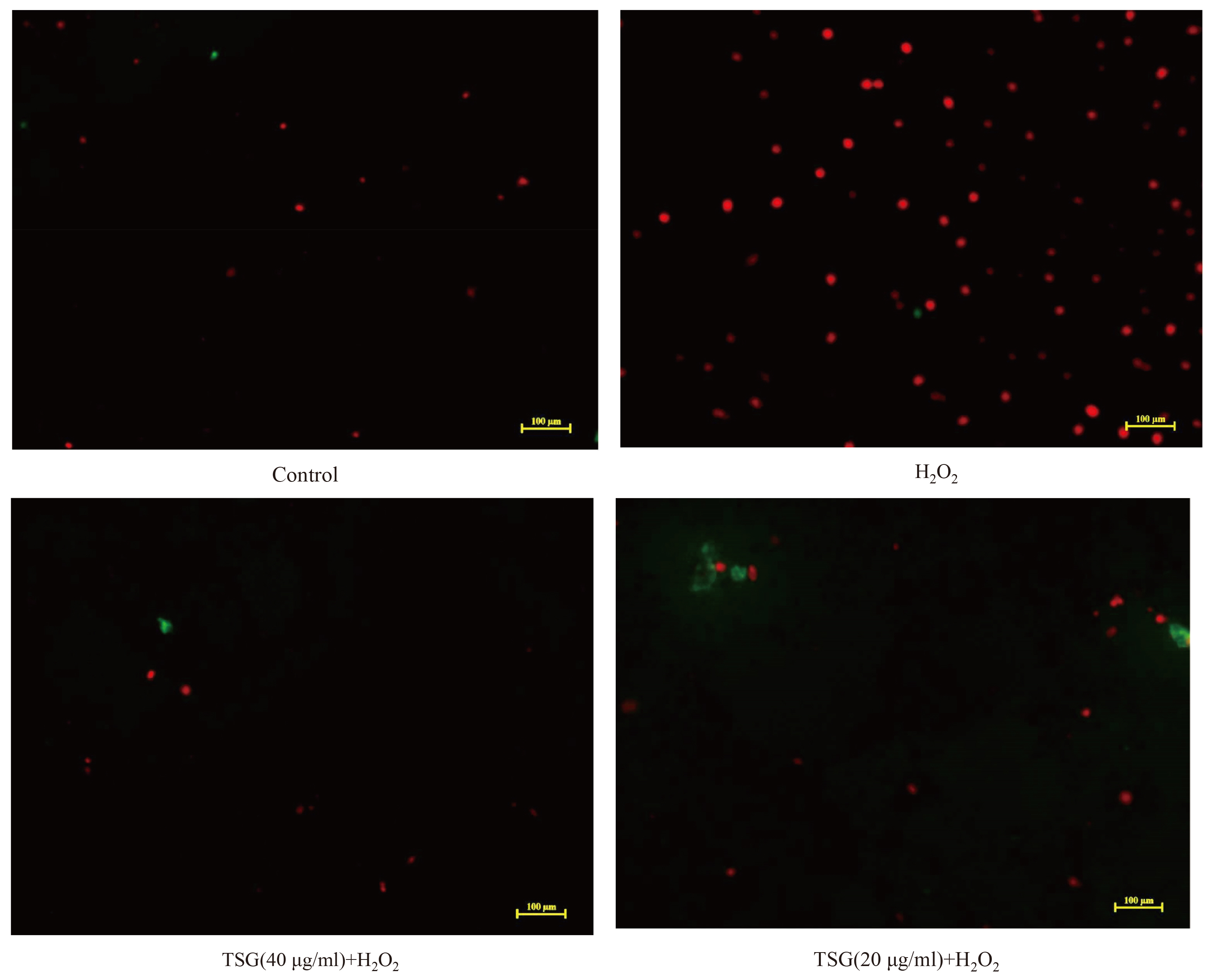
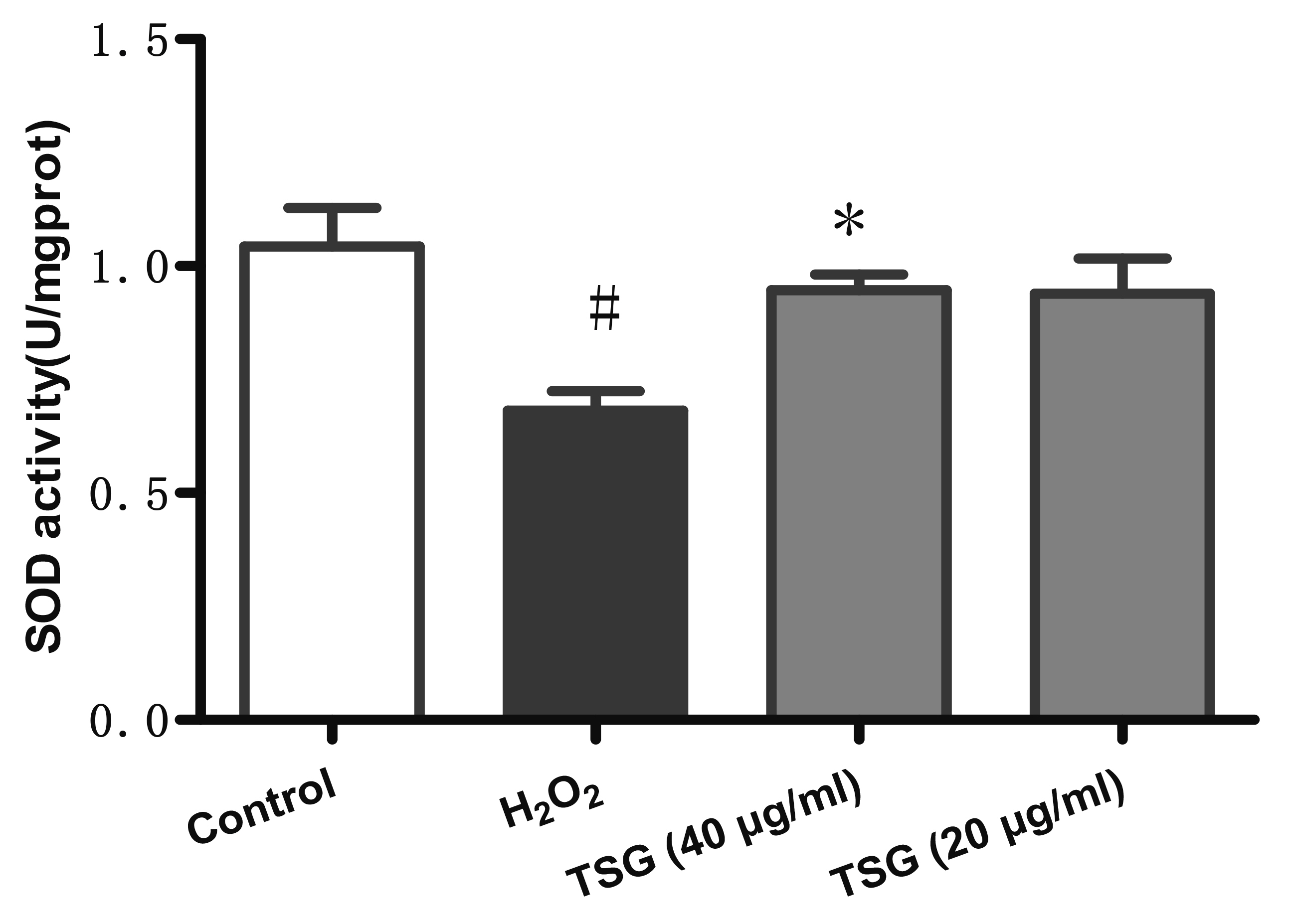
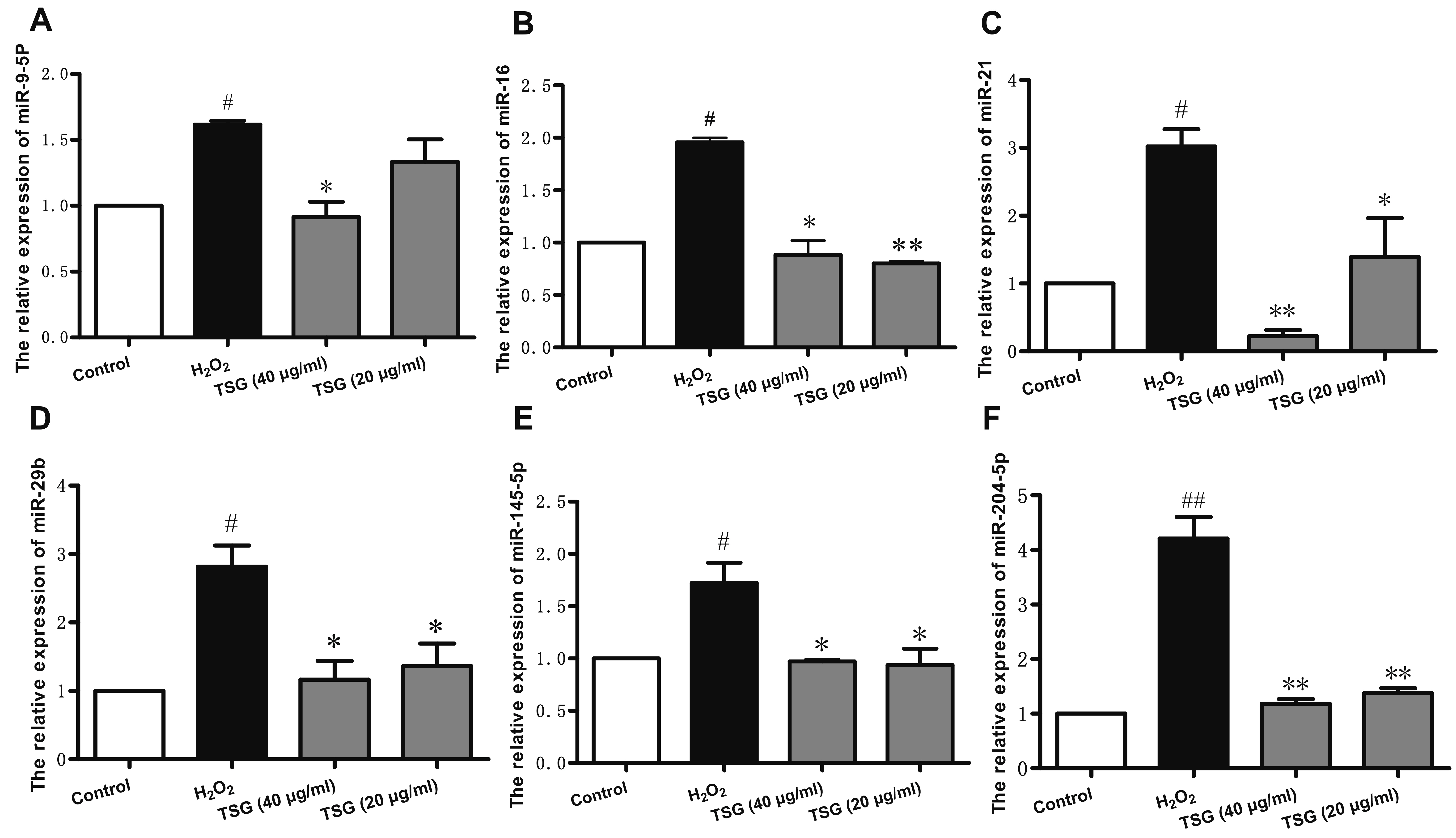
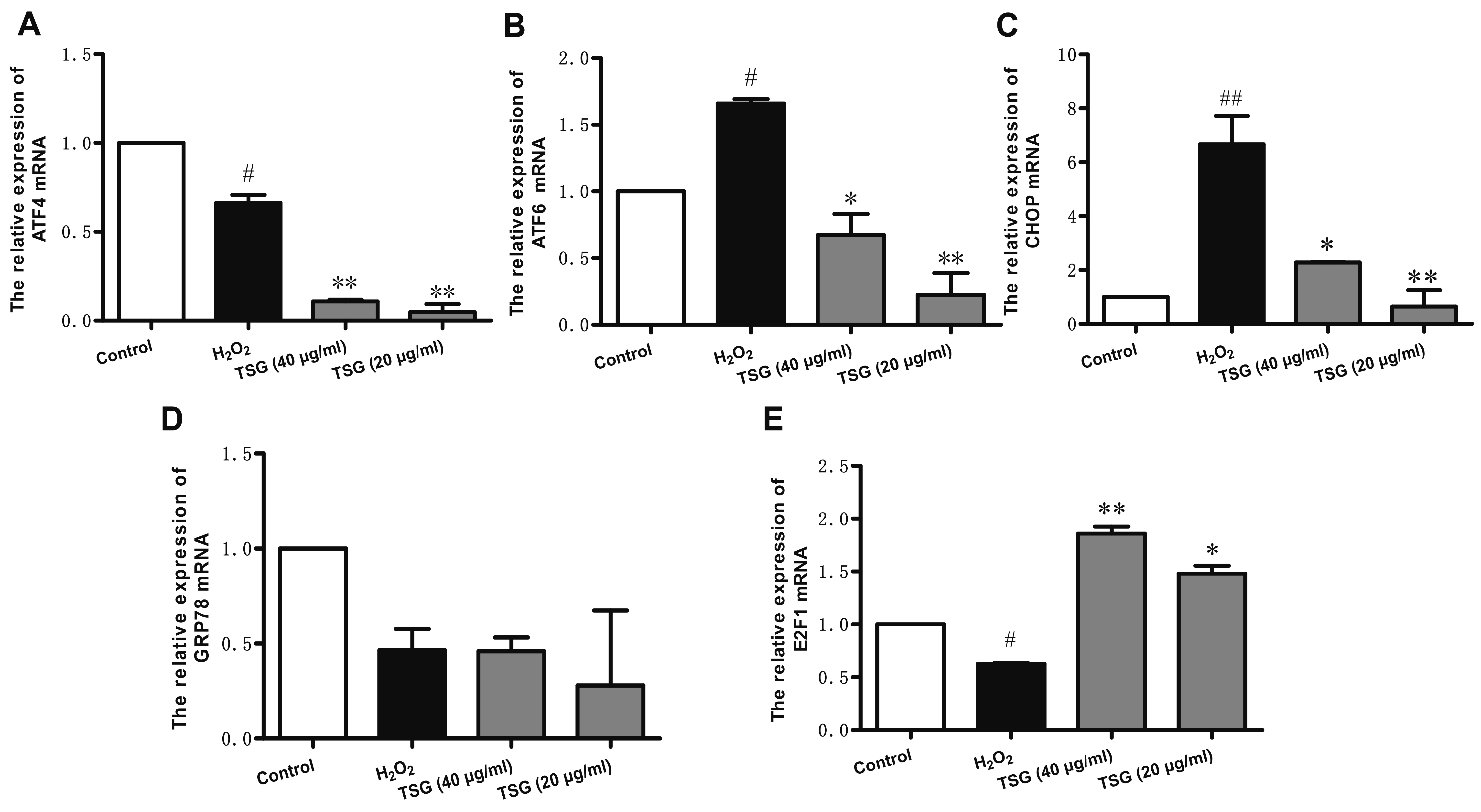
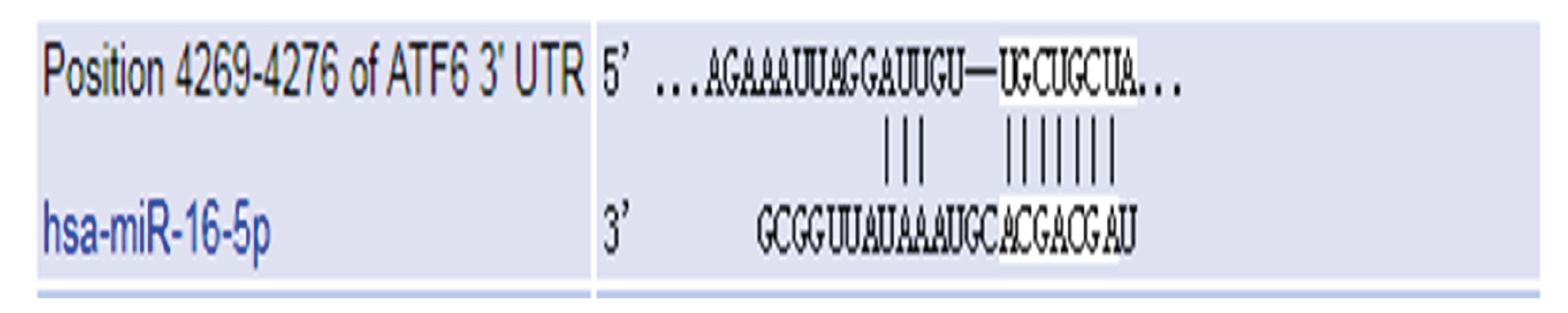
- Endothelial cell injury is a major contributor to cardiovascular diseases. The 2,3,5,4’-Tetrahydroxystilbene-2-O-β-D-Glucoside (TSG) contributes to alleviate human umbilical vein endothelial cells (HUVECs) injury through mechanisms still know a little. This study aims to clarify the TSG effects on gene expression (mRNA and microRNA) related to oxidative stress and endoplasmic reticulum stress induced by H2O2 in HUVECs. We found that TSG significantly reduced the death rate of cells and increased intracellular superoxide dismutase activity. At qRT-PCR, experimental data showed that TSG significantly counteracted the expressions of miR-9-5p, miR-16, miR-21, miR-29b, miR-145-5p, and miR-204-5p. Besides, TSG prevented the expression of ATF6 and CHOP increasing. In contrast, TSG promoted the expression of E2F1. In conclusion, our results point to the obvious protective effect of TSG on HUVECs injury induced by H2O2, and the mechanism may through miR16/ATF6/ E2F1 signaling pathway.
Keyword
Figure
Reference
-
1. Ueda P, Woodward M, Lu Y, Hajifathalian K, Al-Wotayan R, Aguilar-Salinas CA, Ahmadvand A, Azizi F, Bentham J, Cifkova R, Di Cesare M, Eriksen L, Farzadfar F, Ferguson TS, Ikeda N, Khalili D, Khang YH, Lanska V, León-Muñoz L, Magliano DJ, et al. 2017; Laboratory-based and office-based risk scores and charts to predict 10-year risk of cardiovascular disease in 182 countries: a pooled analysis of prospective cohorts and health surveys. Lancet Diabetes Endocrinol. 5:196–213. DOI: 10.1016/S2213-8587(17)30015-3. PMID: 28126460. PMCID: PMC5354360.
Article2. Scioli MG, Storti G, D'Amico F, Rodríguez Guzmán R, Centofanti F, Doldo E, Céspedes Miranda EM, Orlandi A. 2020; Oxidative stress and new pathogenetic mechanisms in endothelial dysfunction: potential diagnostic biomarkers and therapeutic targets. J Clin Med. 9:1995. DOI: 10.3390/jcm9061995. PMID: 32630452. PMCID: PMC7355625.
Article3. Wu Q, Liu XX, Lu DY, Li YT, Sun J, Lan YY, Liu T. 2018; [Protective effect of Polygonum orientale flower extract on H₂O₂-induced oxidative damage of HUVEC cells]. Zhongguo Zhong Yao Za Zhi. 43:1008–1013. Chinese. DOI: 10.19540/j.cnki.cjcmm.2018.0033. PMID: 29676101.4. Büchter C, Zhao L, Havermann S, Honnen S, Fritz G, Proksch P, Wätjen W. 2015; TSG (2,3,5,4'-Tetrahydroxystilbene-2-O-β-D-glucoside) from the Chinese herb Polygonum multiflorum increases life span and stress resistance of Caenorhabditis elegans. Oxid Med Cell Longev. 2015:124357. DOI: 10.1155/2015/124357. PMID: 26075030. PMCID: PMC4436517.5. Zhao J, Liang Y, Song F, Xu S, Nian L, Zhou X, Wang S. 2016; TSG attenuates LPC-induced endothelial cells inflammatory damage through notch signaling inhibition. IUBMB Life. 68:37–50. DOI: 10.1002/iub.1458. PMID: 26662286.
Article6. Zhao J, Xu S, Song F, Nian L, Zhou X, Wang S. 2014; 2,3,5,4'-tetrahydroxystilbene-2-O-β-D-glucoside protects human umbilical vein endothelial cells against lysophosphatidylcholine-induced apoptosis by upregulating superoxide dismutase and glutathione peroxidase. IUBMB Life. 66:711–722. DOI: 10.1002/iub.1321. PMID: 25382724.
Article7. Lee SY, Ahn SM, Wang Z, Choi YW, Shin HK, Choi BT. 2017; Neuroprotective effects of 2,3,5,4'-tetrahydoxystilbene-2-O-β-D-glucoside from Polygonum multiflorum against glutamate-induced oxidative toxicity in HT22 cells. J Ethnopharmacol. 195:64–70. DOI: 10.1016/j.jep.2016.12.001. PMID: 27939422.
Article8. Xie M, Zhang G, Yin W, Hei XX, Liu T. 2018; Cognitive enhancing and antioxidant effects of tetrahydroxystilbene glucoside in Aβ1-42-induced neurodegeneration in mice. J Integr Neurosci. 17:355–365. DOI: 10.3233/JIN-170059. PMID: 29125494.
Article9. Li W, Sun R, Zhou S, Ma J, Xie Y, Xu B, Long H, Luo K, Fang K. 2018; 2,3,5,4'-Tetrahydroxystilbene-2-O-β-D-glucoside inhibits septic serum-induced inflammatory injury via interfering with the ROS-MAPK-NF-κB signaling pathway in pulmonary aortic endothelial cells. Int J Mol Med. 41:1643–1650. DOI: 10.3892/ijmm.2017.3329.10. Di Marzo N, Chisci E, Giovannoni R. 2018; The role of hydrogen peroxide in redox-dependent signaling: homeostatic and pathological responses in mammalian cells. Cells. 7:156. DOI: 10.3390/cells7100156. PMID: 30287799. PMCID: PMC6211135.
Article11. Kuosmanen SM, Kansanen E, Sihvola V, Levonen AL. 2017; MicroRNA profiling reveals distinct profiles for tissue-derived and cultured endothelial cells. Sci Rep. 7:10943. DOI: 10.1038/s41598-017-11487-4. PMID: 28887500. PMCID: PMC5591252.
Article12. Incalza MA, D'Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. 2018; Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 100:1–19. DOI: 10.1016/j.vph.2017.05.005. PMID: 28579545.
Article13. Nemecz M, Alexandru N, Tanko G, Georgescu A. 2016; Role of microRNA in endothelial dysfunction and hypertension. Curr Hypertens Rep. 18:87. DOI: 10.1007/s11906-016-0696-8. PMID: 27837398. PMCID: PMC7102349.
Article14. Yi J, Gao ZF. 2019; MicroRNA-9-5p promotes angiogenesis but inhibits apoptosis and inflammation of high glucose-induced injury in human umbilical vascular endothelial cells by targeting CXCR4. Int J Biol Macromol. 130:1–9. DOI: 10.1016/j.ijbiomac.2019.02.003. PMID: 30716366.
Article15. Sun CY, She XM, Qin Y, Chu ZB, Chen L, Ai LS, Zhang L, Hu Y. 2013; miR-15a and miR-16 affect the angiogenesis of multiple myeloma by targeting VEGF. Carcinogenesis. 34:426–435. DOI: 10.1093/carcin/bgs333. PMID: 23104180.
Article16. Chung HJ, Choi YE, Kim ES, Han YH, Park MJ, Bae IH. 2015; miR-29b attenuates tumorigenicity and stemness maintenance in human glioblastoma multiforme by directly targeting BCL2L2. Oncotarget. 6:18429–18444. DOI: 10.18632/oncotarget.4384. PMID: 26155940. PMCID: PMC4621901.17. Chao J, Guo Y, Li P, Chao L. 2017; Role of kallistatin treatment in aging and cancer by modulating miR-34a and miR-21 expression. Oxid Med Cell Longev. 2017:5025610. DOI: 10.1155/2017/5025610. PMID: 28744338. PMCID: PMC5506461.
Article18. Pan Y, Ye C, Tian Q, Yan S, Zeng X, Xiao C, Wang L, Wang H. 2018; miR-145 suppresses the proliferation, invasion and migration of NSCLC cells by regulating the BAX/BCL-2 ratio and the caspase-3 cascade. Oncol Lett. 15:4337–4343. DOI: 10.3892/ol.2018.7863. PMID: 29541201. PMCID: PMC5835894.
Article19. Liu X, Gao X, Zhang W, Zhu T, Bi W, Zhang Y. 2018; MicroRNA-204 deregulation in lung adenocarcinoma controls the biological behaviors of endothelial cells potentially by modulating Janus kinase 2-signal transducer and activator of transcription 3 pathway. IUBMB Life. 70:81–91. DOI: 10.1002/iub.1706. PMID: 29281186.
Article20. Rutkowski DT, Kaufman RJ. 2004; A trip to the ER: coping with stress. Trends Cell Biol. 14:20–28. DOI: 10.1016/j.tcb.2003.11.001. PMID: 14729177.
Article21. Zhang JJ, Zhang YZ, Peng JJ, Li NS, Xiong XM, Ma QL, Luo XJ, Liu B, Peng J. 2018; Atorvastatin exerts inhibitory effect on endothelial senescence in hyperlipidemic rats through a mechanism involving down-regulation of miR-21-5p/203a-3p. Mech Ageing Dev. 169:10–18. DOI: 10.1016/j.mad.2017.12.001. PMID: 29248491.
Article22. Zhang M, Zhou SH, Li XP, Shen XQ, Fang ZF, Liu QM, Qiu SF, Zhao SP. 2008; Atorvastatin downregulates BMP-2 expression induced by oxidized low-density lipoprotein in human umbilical vein endothelial cells. Circ J. 72:807–812. DOI: 10.1253/circj.72.807. PMID: 18441463.
Article23. Yi TN, Zhao HY, Zhang JS, Shan HY, Meng X, Zhang J. 2009; Effect of aspirin on high glucose-induced senescence of endothelial cells. Chin Med J (Engl). 122:3055–3061. PMID: 20137501.24. Pagliarini V, Giglio P, Bernardoni P, De Zio D, Fimia GM, Piacentini M, Corazzari M. 2015; Downregulation of E2F1 during ER stress is required to induce apoptosis. J Cell Sci. 128:1166–1179. DOI: 10.1242/jcs.164103. PMID: 25616897.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The expression of heat shock protein (hsp) 90 and 27 in human umbilical vein endothelial cell (HUVEC)
- Apoptotic Effect of Radiocontrast Media on Human Umbilical Vein Endothelial Cells
- Propofol attenuates hydrogenperoxide-induced apoptosis in human umbilical vein endothelial cells via multiple signaling pathways
- In Vitro Culture of Endothelial Cell and Smooth Muscle Cell for Studying Vascular Diseases
- Aspirin-Triggered Resolvin D1 Inhibits TGF-β1-Induced EndMT through Increasing the Expression of Smad7 and Is Closely Related to Oxidative Stress