Korean J Ophthalmol.
2020 Apr;34(2):150-157. 10.3341/kjo.2019.0120.
Association between Ranibizumab Injections and Risk of Acute Myocardial Infarction in Age-related Macular Degeneration: A Case-crossover Study
- KMID: 2507408
- DOI: http://doi.org/10.3341/kjo.2019.0120
Abstract
- Purpose
This study aimed to evaluate the risk of acute myocardial infarction (AMI) associated with intravitreal ranibizumab in age-related macular degeneration (AMD).
Methods
This nationwide retrospective case-crossover study using data from the Korean National Health Insurance Service database included patients diagnosed with exudative AMD using the registration code for exudative AMD (V201) from 2009 to 2014. We identified all incident AMI cases among these exudative AMD cases from inpatient claims and defined the index date as the date of hospitalization. For each patient, we defined the case period as one to 60 days and four control periods as 121 to 180, 181 to 240, 241 to 300, and 301 to 361 days, respectively, before the index date. A prescription of ranibizumab was searched for during the case and control periods. We calculated the adjusted odds ratios and their 95% confidence intervals using a conditional logistic regression model.
Results
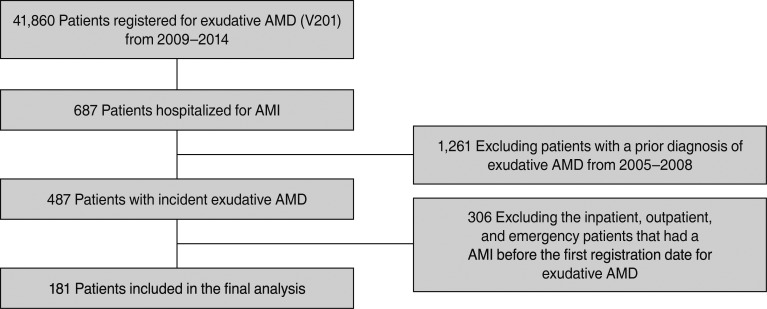
From a cohort of patients with exudative AMD (n = 41,860), a total of 181 AMI patients with exudative AMD were included. Among all the patients, 11.05% were treated during the 2 months preceding the index date as compared with 8.29% to 9.39% treated during control periods. The adjusted odds ratio of AMI associated with intravitreal ranibizumab during the preceding 2 months was 1.22 (95% confidence interval, 0.673–2.213; p = 0.5124). Analyses based on case periods of 15 days and 1 month yielded similar results.
Conclusions
Intravitreal ranibizumab injection does not appear to increase the risk of hospitalization for AMI within 60 days in exudative AMD patients.
Figure
Reference
-
1. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–1431. PMID: 17021318.
Article2. Frank RN, Amin RH, Eliott D, et al. Basic fibroblast growth factor and vascular endothelial growth factor are present in epiretinal and choroidal neovascular membranes. Am J Ophthalmol. 1996; 122:393–403. PMID: 8794712.
Article3. Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996; 37:1929–1934. PMID: 8759365.
Article4. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–1444. PMID: 17021319.
Article5. Simons M. Angiogenesis: where do we stand now? Circulation. 2005; 111:1556–1566. PMID: 15795364.6. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997; 18:4–25. PMID: 9034784.
Article7. Henry TD, Annex BH, McKendall GR, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003; 107:1359–1365. PMID: 12642354.8. Juan-Babot JO, Martinez-Gonzalez J, Berrozpe M, Badimon L. Neovascularization in human coronary arteries with lesions of different severity. Rev Esp Cardiol. 2003; 56:978–986. PMID: 14563292.9. Tunon J, Ruiz-Moreno JM, Martin-Ventura JL, et al. Cardiovascular risk and antiangiogenic therapy for age-related macular degeneration. Surv Ophthalmol. 2009; 54:339–348. PMID: 19422962.10. Kemp A, Preen DB, Morlet N, et al. Myocardial infarction after intravitreal vascular endothelial growth factor inhibitors: a whole population study. Retina. 2013; 33:920–927. PMID: 23492942.11. Schlenker MB, Thiruchelvam D, Redelmeier DA. Intravitreal anti-vascular endothelial growth factor treatment and the risk of thromboembolism. Am J Ophthalmol. 2015; 160:569–580. PMID: 26116264.
Article12. Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009; 116:57–65. PMID: 19118696.
Article13. Abraham P, Yue H, Wilson L. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 2. Am J Ophthalmol. 2010; 150:315–324. PMID: 20598667.
Article14. Antoszyk AN, Tuomi L, Chung CY, et al. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration (FOCUS): year 2 results. Am J Ophthalmol. 2008; 145:862–874. PMID: 18321465.
Article15. Wong TY, Tikellis G, Sun C, et al. Age-related macular degeneration and risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Ophthalmology. 2007; 114:86–91. PMID: 17198851.16. Wong TY. Age-related macular degeneration and cardiovascular disease in the era of anti-vascular endothelial growth factor therapies. Am J Ophthalmol. 2009; 148:327–329. PMID: 19703607.
Article17. Boyer DS, Heier JS, Brown DM, et al. A Phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology. 2009; 116:1731–1739. PMID: 19643495.
Article18. Busbee BG, Ho AC, Brown DM, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013; 120:1046–1056. PMID: 23352196.
Article19. Schmidt-Erfurth U, Eldem B, Guymer R, et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology. 2011; 118:831–839. PMID: 21146229.
Article20. IVAN Study Investigators. Chakravarthy U, Harding SP, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012; 119:1399–1411. PMID: 22578446.21. Ueta T, Noda Y, Toyama T, et al. Systemic vascular safety of ranibizumab for age-related macular degeneration: systematic review and meta-analysis of randomized trials. Ophthalmology. 2014; 121:2193–2203. PMID: 25023760.22. Ueta T, Yanagi Y, Tamaki Y, Yamaguchi T. Cerebrovascular accidents in ranibizumab. Ophthalmology. 2009; 116:362.
Article23. Curtis LH, Hammill BG, Schulman KA, Cousins SW. Risks of mortality, myocardial infarction, bleeding, and stroke associated with therapies for age-related macular degeneration. Arch Ophthalmol. 2010; 128:1273–1279. PMID: 20937996.
Article24. Pratt NL, Ramsay EN, Kemp A, et al. Ranibizumab and risk of hospitalisation for ischaemic stroke and myocardial infarction in patients with age-related macular degeneration: a self-controlled case-series analysis. Drug Saf. 2014; 37:1021–1027. PMID: 25260802.
Article25. Kimm H, Yun JE, Lee SH, et al. Validity of the diagnosis of acute myocardial infarction in korean national medical health insurance claims data: the korean heart study (1). Korean Circ J. 2012; 42:10–15. PMID: 22363378.
Article26. Park JK, Kim KS, Kim CB, et al. The accuracy of ICD codes for cerebrovascular diseases in medical insurance claims. Korean J Prev Med. 2000; 33:76–82.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intravitreal Ranibizumab Therapy for Neovascular Age-Related Macular Degeneration with a Predominantly Hemorrhagic Lesion
- Efficacy of Three Aflibercept Injections for Neovascular Age-related Macular Degeneration Showing Limited Response to Ranibizumab
- Development of Subretinal Hemorrhage during Treatment of Neovascular Age-related Macular Degeneration Using a Treat-and-extend Regimen: A Case Report
- Clinical Changes after Switching from Ranibizumab/Aflibercept to Bevacizumab in Exudative Age-related Macular Degeneration
- Macular Hole Following Intravitreal Ranibizumab Injections for Choroidal Neovascularization