Korean Circ J.
2020 Oct;50(10):907-922. 10.4070/kcj.2020.0061.
Comparing the Procedural and Clinical Outcomes of Sapien XT and Sapien 3 Valves in Transcatheter Aortic Valve Replacement in Korean Patients
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 2Division of Cardiology, Department of Internal Medicine, Yuseong Sun Hospital, Daejeon, Korea
- 3Department of Biostatistics, Korea University College of Medicine, Seoul, Korea
- 4Division of Cardiology, Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University College of Medicine, Seoul, Korea
- 5Division of Cardiology, Department of Internal Medicine, Sejong Heart Institute, Sejong General Hospital, Bucheon, Korea
- 6Division of Cardiology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2507008
- DOI: http://doi.org/10.4070/kcj.2020.0061
Abstract
- Background and Objectives
The Sapien 3 (S3) valve has not been compared to the Sapien XT (SXT) valve in Korea. We compared procedural and clinical outcomes between the 2 devices.
Methods
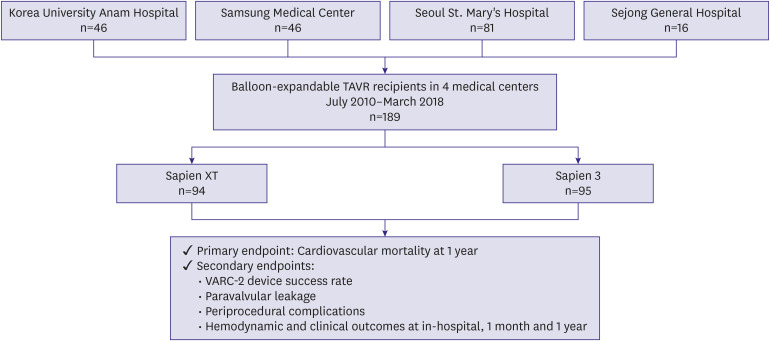
A total of 189 patients who underwent transcatheter aortic valve replacement (TAVR) with S3 (n=95) or SXT (n=94) valve was analyzed. The primary endpoint was cardiovascular mortality at 1 year. The median follow-up duration was 438 days.
Results
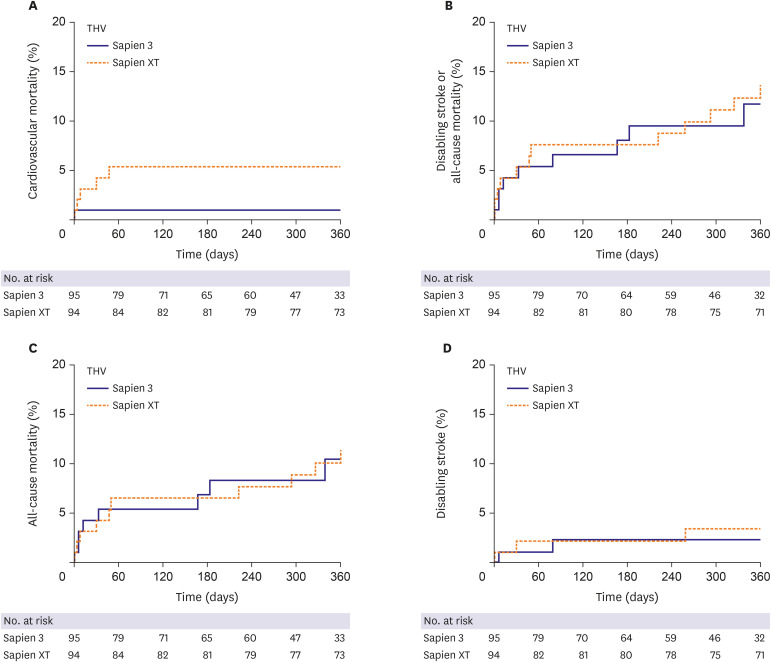
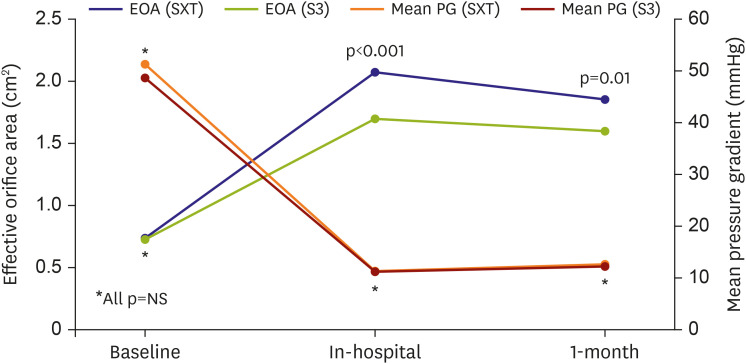
The Society of Thoracic Surgeons score was similar between the 2 groups. The device success rate (90.4% vs. 97.9%; p=0.028) was higher in the S3 than in the SXT. The S3 showed significantly fewer cases of moderate or severe paravalvular leakage (PVL) (16.7% vs. 0.0%; p=0.001) than the SXT. However, effective orifice area (EOA) (2.07±0.61 vs. 1.70±0.49 cm2 ; p<0.001) was smaller in the S3. Multivariable Cox regression analysis showed the S3 was associated with significantly fewer cardiovascular mortality at 1 year compared to the SXT (5.4% vs. 1.1%; hazard ratio, 0.031; 95% confidence interval, 0.001–0.951; p=0.047). Periprocedural complication rates, composite of disabling stroke or all-cause mortality, allcause mortality, and disabling stroke at 1 year were similar between the 2 groups.
Conclusions
Cardiovascular mortality was lower in the S3 group than in the SXT group over 1 year of follow-up. The reduction in PVL was attributed to the higher device success rate of TAVR with the S3 valve. However, the benefit of S3 obtained at the expense of reduced EOA should be meticulously re-evaluated in larger studies during long-term follow-up.
Figure
Cited by 1 articles
-
Sapien 3 versus Sapien XT Balloon-expanding Valve for Transcatheter Aortic Valve Replacement: Improved Immediate and Late Outcomes at the Expense of Smaller Effective Orifice Area
Young-Guk Ko
Korean Circ J. 2020;50(10):923-924. doi: 10.4070/kcj.2020.0353.
Reference
-
1. Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017; 38:2739–2791. PMID: 28886619.2. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2017; 70:252–289. PMID: 28315732.3. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017; 376:1321–1331. PMID: 28304219.4. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016; 374:1609–1620. PMID: 27040324.5. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019; 380:1695–1705. PMID: 30883058.6. Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019; 380:1706–1715. PMID: 30883053.7. Yu CW, Kim WJ, Ahn JM, et al. Trends and outcomes of transcatheter aortic valve implantation (TAVI) in Korea: the results of the first cohort of Korean TAVI registry. Korean Circ J. 2018; 48:382–394. PMID: 29671283.
Article8. Van Belle E, Juthier F, Susen S, et al. Postprocedural aortic regurgitation in balloon-expandable and self-expandable transcatheter aortic valve replacement procedures: analysis of predictors and impact on long-term mortality: insights from the FRANCE2 registry. Circulation. 2014; 129:1415–1427. PMID: 24566199.9. Kazuno Y, Maeno Y, Kawamori H, et al. Comparison of Sapien 3 and Sapien XT transcatheter heart valve stent-frame expansion: evaluation using multi-slice computed tomography. Eur Heart J Cardiovasc Imaging. 2016; 17:1054–1062. PMID: 27002141.
Article10. Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol. 2007; 50:69–76. PMID: 17601548.11. Sawa Y, Saito S, Kobayashi J, et al. First clinical trial of a self-expandable transcatheter heart valve in Japan in patients with symptomatic severe aortic stenosis. Circ J. 2014; 78:1083–1090. PMID: 24662399.
Article12. Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012; 33:2403–2418. PMID: 23026477.
Article13. Vendrik J, van Kesteren F, van Mourik MS, et al. Procedural outcome and midterm survival of lower risk transfemoral transcatheter aortic valve implantation patients treated with the Sapien XT or Sapien 3 device. Am J Cardiol. 2018; 121:856–861. PMID: 29415808.
Article14. Nijhoff F, Abawi M, Agostoni P, Ramjankhan FZ, Doevendans PA, Stella PR. Transcatheter aortic valve implantation with the new balloon-expandable Sapien 3 versus Sapien XT valve system: a propensity score-matched single-center comparison. Circ Cardiovasc Interv. 2015; 8:e002408. PMID: 26033967.
Article15. Sinning JM, Hammerstingl C, Vasa-Nicotera M, et al. Aortic regurgitation index defines severity of peri-prosthetic regurgitation and predicts outcome in patients after transcatheter aortic valve implantation. J Am Coll Cardiol. 2012; 59:1134–1141. PMID: 22440213.
Article16. Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011; 123:299–308. PMID: 21220731.
Article17. Lefèvre T, Kappetein AP, Wolner E, et al. One year follow-up of the multi-centre European PARTNER transcatheter heart valve study. Eur Heart J. 2011; 32:148–157. PMID: 21075775.18. Leipsic J, Gurvitch R, Labounty TM, et al. Multidetector computed tomography in transcatheter aortic valve implantation. JACC Cardiovasc Imaging. 2011; 4:416–429. PMID: 21492818.
Article19. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010; 363:1597–1607. PMID: 20961243.20. Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014; 370:1790–1798. PMID: 24678937.
Article21. Ahmad M, Patel JN, Kim M, Baman T, Barzallo M, Mungee S. Larger valve size is associated with permanent pacemaker implantation in edwards Sapien 3™ transcatheter aortic valves. Cureus. 2019; 11:e4370. PMID: 31192075.
Article22. Bax JJ, Delgado V, Bapat V, et al. Open issues in transcatheter aortic valve implantation. Part 2: procedural issues and outcomes after transcatheter aortic valve implantation. Eur Heart J. 2014; 35:2639–2654. PMID: 25062953.
Article23. Binder RK, Stortecky S, Heg D, et al. Procedural results and clinical outcomes of transcatheter aortic valve implantation in Switzerland: an observational cohort study of Sapien 3 versus Sapien XT transcatheter heart valves. Circ Cardiovasc Interv. 2015; 8:e002653. PMID: 26453687.
Article24. Rahimtoola SH. The problem of valve prosthesis-patient mismatch. Circulation. 1978; 58:20–24. PMID: 348341.
Article25. Pibarot P, Dumesnil JG. Prosthesis-patient mismatch: definition, clinical impact, and prevention. Heart. 2006; 92:1022–1029. PMID: 16251232.
Article26. Theron A, Pinto J, Grisoli D, et al. Patient-prosthesis mismatch in new generation trans-catheter heart valves: a propensity score analysis. Eur Heart J Cardiovasc Imaging. 2018; 19:225–233. PMID: 28329317.
Article27. Tuzcu EM, Kapadia SR, Vemulapalli S, et al. Transcatheter aortic valve replacement of failed surgically implanted bioprostheses: the STS/ACC registry. J Am Coll Cardiol. 2018; 72:370–382. PMID: 30025572.28. Webb JG, Murdoch DJ, Alu MC, et al. 3-year outcomes after valve-in-valve transcatheter aortic valve replacement for degenerated bioprostheses: the PARTNER 2 registry. J Am Coll Cardiol. 2019; 73:2647–2655. PMID: 31146808.29. Dvir D, Webb JG, Bleiziffer S, et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014; 312:162–170. PMID: 25005653.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sapien 3 versus Sapien XT Balloonexpanding Valve for Transcatheter Aortic Valve Replacement: Improved Immediate and Late Outcomes at the Expense of Smaller Effective Orifice Area
- Leadless Pacemaker Implantation Following Transcatheter Aortic Valve Implantation Using SAPIEN 3
- Transcatheter Mitral Valve Implantation in Open Heart Surgery: An Off-Label Technique
- Expanding transcatheter aortic valve replacement into uncharted indications
- Comparable Outcomes of Bicuspid Aortic Valves for RapidDeployment Aortic Valve Replacement