J Korean Med Sci.
2020 Sep;35(36):e305. 10.3346/jkms.2020.35.e305.
Beneficial Effect of Chloroquine and Amodiaquine on Type 1 Diabetic Tubulopathy by Attenuating Mitochondrial Nox4 and Endoplasmic Reticulum Stress
- Affiliations
-
- 1Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea
- 2Genomics Core Facility, Department of Transdisciplinary Research and Collaboration, Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea
- 3Department of Internal Medicine, Kyung Hee University Hospital at Gangdong, Kyung Hee University School of Medicine, Seoul, Korea
- KMID: 2506545
- DOI: http://doi.org/10.3346/jkms.2020.35.e305
Abstract
- Background
Oxidative stress induced by chronic hyperglycemia is recognized as a significant mechanistic contributor to the development of diabetic kidney disease (DKD). Nonphagocytic nicotinamide adenine dinucleotide phosphate oxidase 4 (Nox4) is a major source of reactive oxygen species (ROS) in many cell types and in the kidney tissue of diabetic animals. We designed this study to explore the therapeutic potential of chloroquine (CQ) and amodiaquine (AQ) for inhibiting mitochondrial Nox4 and diabetic tubular injury.
Methods
Human renal proximal tubular epithelial cells (hRPTCs) were cultured in highglucose media (30 mM D-glucose), and diabetes was induced with streptozotocin (STZ, 50 mg/kg i.p. for 5 days) in male C57BL/6J mice. CQ and AQ were administered to the mice via intraperitoneal injection for 14 weeks.
Results
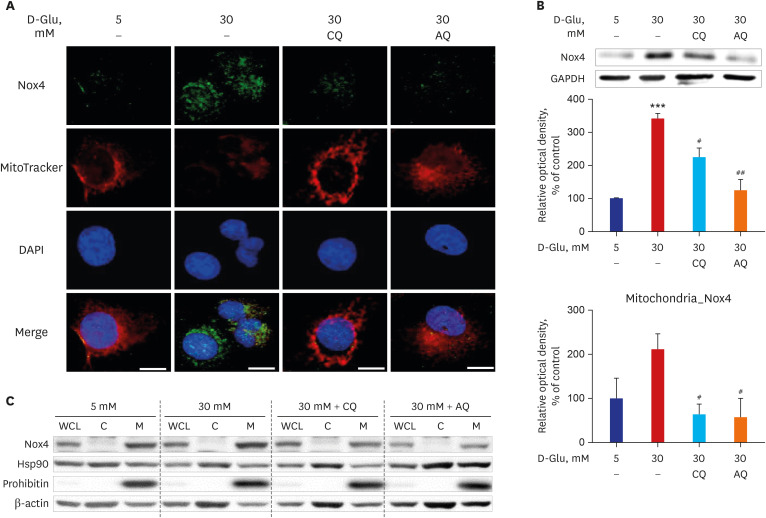
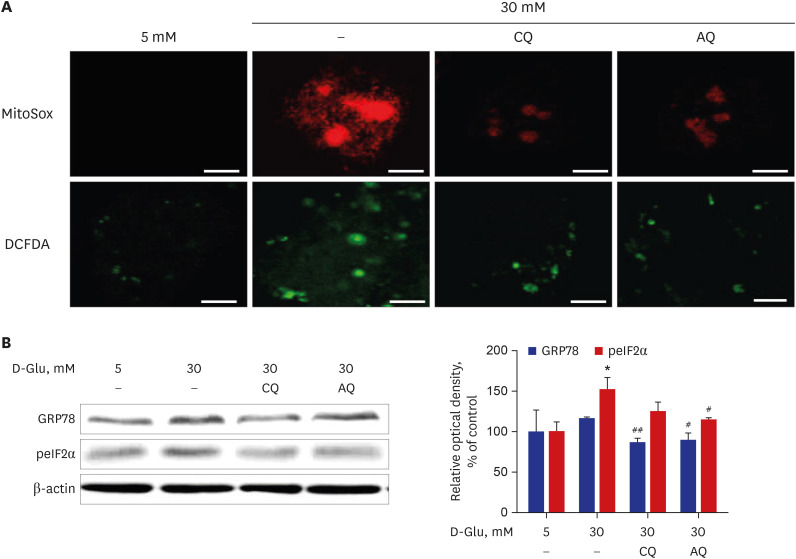
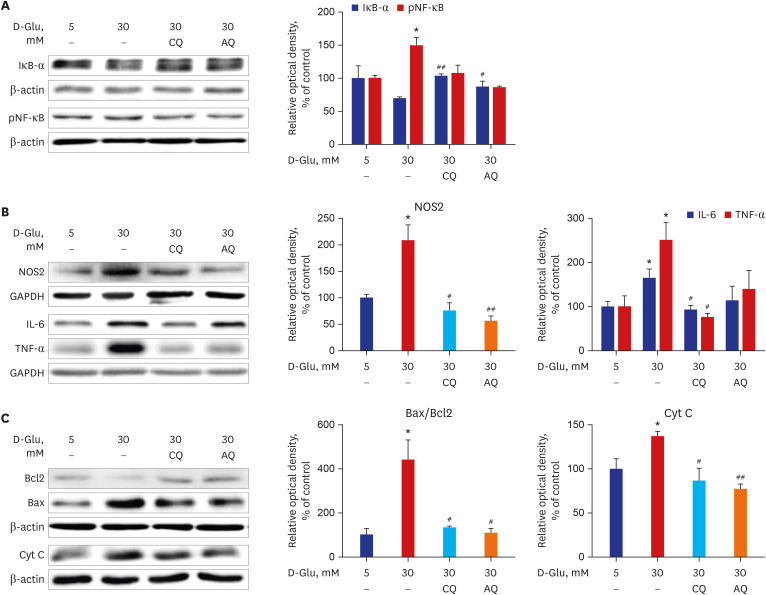
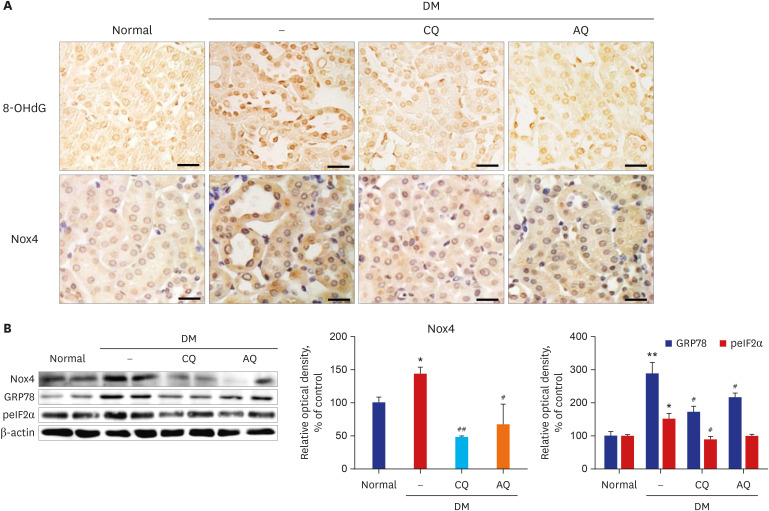
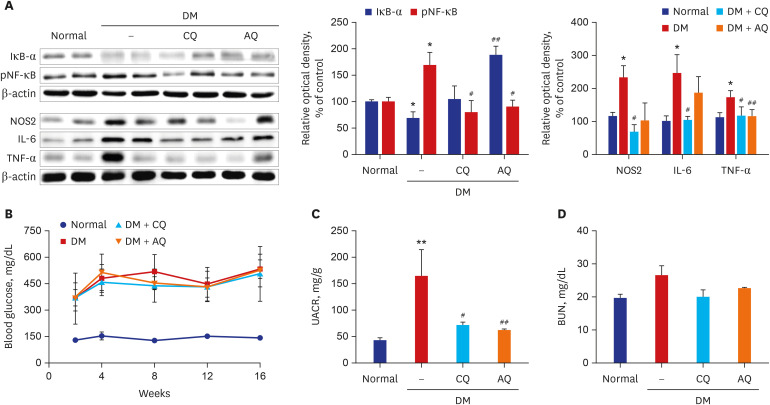
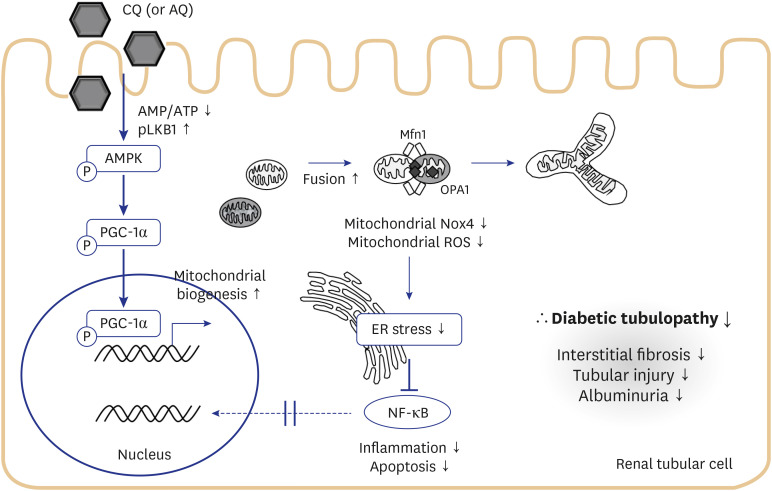
CQ and AQ inhibited mitochondrial Nox4 and increased mitochondrial mass in hRPTCs under high-glucose conditions. Reduced mitochondrial ROS production after treatment with the drugs resulted in decreased endoplasmic reticulum (ER) stress, suppressed inflammatory protein expression and reduced cell apoptosis in hRPTCs under high-glucose conditions. Notably, CQ and AQ treatment diminished Nox4 activation and ER stress in the kidneys of STZ-induced diabetic mice. In addition, we observed attenuated inflammatory protein expression and albuminuria in STZ-induced diabetic mice after CQ and AQ treatment.
Conclusion
We substantiated the protective actions of CQ and AQ in diabetic tubulopathy associated with reduced mitochondrial Nox4 activation and ER stress alleviation. Further studies exploring the roles of mitochondrial Nox4 in the pathogenesis of DKD could suggest new therapeutic targets for patients with DKD.
Keyword
Figure
Reference
-
1. Jha JC, Gray SP, Barit D, Okabe J, El-Osta A, Namikoshi T, et al. Genetic targeting or pharmacologic inhibition of NADPH oxidase Nox4 provides renoprotection in long-term diabetic nephropathy. J Am Soc Nephrol. 2014; 25(6):1237–1254. PMID: 24511132.
Article2. Tonelli M, Sacks F, Pfeffer M, Jhangri GS, Curhan G. Cholesterol and Recurrent Events (CARE) Trial Investigators. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005; 68(1):237–245. PMID: 15954913.3. Okamura DM, Himmelfarb J. Tipping the redox balance of oxidative stress in fibrogenic pathways in chronic kidney disease. Pediatr Nephrol. 2009; 24(12):2309–2319. PMID: 19421784.
Article4. Xiao H, Li Y, Qi J, Wang H, Liu K. Peroxynitrite plays a key role in glomerular lesions in diabetic rats. J Nephrol. 2009; 22(6):800–808. PMID: 19967660.5. Kanauchi M, Nishioka H, Hashimoto T. Oxidative DNA damage and tubulointerstitial injury in diabetic nephropathy. Nephron. 2002; 91(2):327–329. PMID: 12053073.
Article6. Hinokio Y, Suzuki S, Hirai M, Suzuki C, Suzuki M, Toyota T. Urinary excretion of 8-oxo-7, 8-dihydro-2′-deoxyguanosine as a predictor of the development of diabetic nephropathy. Diabetologia. 2002; 45(6):877–882. PMID: 12107732.
Article7. Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A. 2009; 106(34):14385–14390. PMID: 19706525.
Article8. Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, et al. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005; 280(47):39616–39626. PMID: 16135519.
Article9. Kim SM, Kim YG, Jeong KH, Lee SH, Lee TW, Ihm CG, et al. Angiotensin II-induced mitochondrial Nox4 is a major endogenous source of oxidative stress in kidney tubular cells. PLoS One. 2012; 7(7):e39739. PMID: 22808054.
Article10. Ruiz-Ortega M, Bustos C, Plaza JJ, Egido J. Overexpression of extracellular matrix proteins in renal tubulointerstitial cells by platelet-activating-factor stimulation. Nephrol Dial Transplant. 1998; 13(4):886–892. PMID: 9568845.11. Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, et al. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells. 2005; 10(12):1139–1151. PMID: 16324151.
Article12. Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of Nox2 and Nox4 in primary human endothelial cells. Antioxid Redox Signal. 2005; 7(3-4):308–317. PMID: 15706079.13. Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010; 106(7):1253–1264. PMID: 20185797.
Article14. Jeong HY, Kang JM, Jun HH, Kim DJ, Park SH, Sung MJ, et al. Chloroquine and amodiaquine enhance AMPK phosphorylation and improve mitochondrial fragmentation in diabetic tubulopathy. Sci Rep. 2018; 8(1):8774. PMID: 29884802.
Article15. Kume S, Koya D. Autophagy: a novel therapeutic target for diabetic nephropathy. Diabetes Metab J. 2015; 39(6):451–460. PMID: 26706914.
Article16. Lee SY, Kang JM, Kim DJ, Park SH, Jeong HY, Lee YH, et al. PGC1α activators mitigate diabetic tubulopathy by improving mitochondrial dynamics and quality control. J Diabetes Res. 2017; 2017:6483572. PMID: 28409163.17. Kitamura M. Endoplasmic reticulum stress and unfolded protein response in renal pathophysiology: Janus faces. Am J Physiol Renal Physiol. 2008; 295(2):F323–F334. PMID: 18367660.
Article18. Fan Y, Lee K, Wang N, He JC. The role of endoplasmic reticulum stress in diabetic nephropathy. Curr Diab Rep. 2017; 17(3):17. PMID: 28271468.
Article19. Cameron NE. Role of endoplasmic reticulum stress in diabetic neuropathy. Diabetes. 2013; 62(3):696–697. PMID: 23431013.
Article20. Tang SC, Lai KN. The pathogenic role of the renal proximal tubular cell in diabetic nephropathy. Nephrol Dial Transplant. 2012; 27(8):3049–3056. PMID: 22734110.
Article21. Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D. Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol. 2009; 20(3):489–494. PMID: 19118149.
Article22. Dickson LE, Wagner MC, Sandoval RM, Molitoris BA. The proximal tubule and albuminuria: really! J Am Soc Nephrol. 2014; 25(3):443–453. PMID: 24408874.
Article23. Ernster L, Schatz G. Mitochondria: a historical review. J Cell Biol. 1981; 91(3 Pt 2):227s–255s. PMID: 7033239.
Article24. Bereiter-Hahn J, Vöth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Tech. 1994; 27(3):198–219. PMID: 8204911.
Article25. Nunnari J, Marshall WF, Straight A, Murray A, Sedat JW, Walter P. Mitochondrial transmission during mating in Saccharomyces cerevisiae is determined by mitochondrial fusion and fission and the intramitochondrial segregation of mitochondrial DNA. Mol Biol Cell. 1997; 8(7):1233–1242. PMID: 9243504.
Article26. Yang S, Han Y, Liu J, Song P, Xu X, Zhao L, et al. Mitochondria: a novel therapeutic target in diabetic nephropathy. Curr Med Chem. 2017; 24(29):3185–3202. PMID: 28486920.
Article27. Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol. 2013; 24(11):1901–1912. PMID: 23949796.
Article28. Higgins GC, Coughlan MT. Mitochondrial dysfunction and mitophagy: the beginning and end to diabetic nephropathy? Br J Pharmacol. 2014; 171(8):1917–1942. PMID: 24720258.
Article29. Takebayashi S, Kaneda K. Mitochondrial derangement: possible initiator of microalbuminuria in NIDDM. J Diabet Complications. 1991; 5(2-3):104–106. PMID: 1770011.
Article30. Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest. 2013; 123(11):4888–4899. PMID: 24135141.
Article31. Lee YH, Kim SH, Kang JM, Heo JH, Kim DJ, Park SH, et al. Empagliflozin attenuates diabetic tubulopathy by improving mitochondrial fragmentation and autophagy. Am J Physiol Renal Physiol. 2019; 317(4):F767–F780. PMID: 31390268.
Article32. Sagoo MK, Gnudi L. Diabetic nephropathy: is there a role for oxidative stress? Free Radic Biol Med. 2018; 116:50–63. PMID: 29305106.
Article33. Altenhöfer S, Radermacher KA, Kleikers PW, Wingler K, Schmidt HH. Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxid Redox Signal. 2015; 23(5):406–427. PMID: 24383718.
Article34. Sahoo S, Meijles DN, Pagano PJ. NADPH oxidases: key modulators in aging and age-related cardiovascular diseases? Clin Sci (Lond). 2016; 130(5):317–335. PMID: 26814203.
Article35. Nagasu H, Satoh M, Kiyokage E, Kidokoro K, Toida K, Channon KM, et al. Activation of endothelial NAD(P)H oxidase accelerates early glomerular injury in diabetic mice. Lab Invest. 2016; 96(1):25–36. PMID: 26552047.
Article36. You YH, Okada S, Ly S, Jandeleit-Dahm K, Barit D, Namikoshi T, et al. Role of Nox2 in diabetic kidney disease. Am J Physiol Renal Physiol. 2013; 304(7):F840–F848. PMID: 23389458.
Article37. Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002; 110(10):1389–1398. PMID: 12438434.
Article38. Lindenmeyer MT, Rastaldi MP, Ikehata M, Neusser MA, Kretzler M, Cohen CD, et al. Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J Am Soc Nephrol. 2008; 19(11):2225–2236. PMID: 18776125.
Article39. Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012; 13(2):89–102. PMID: 22251901.
Article40. Cybulsky AV. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat Rev Nephrol. 2017; 13(11):681–696. PMID: 28970584.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoplasmic Reticulum Stress and Diabetes
- Blockade of Autophagy Aggravates Endoplasmic Reticulum Stress and Improves Paclitaxel Cytotoxicity in Human Cervical Cancer Cells
- Nuclear Receptors Resolve Endoplasmic Reticulum Stress to Improve Hepatic Insulin Resistance
- New Insights into the Role of Endoplasmic Reticulum Stress in Breast Cancer Metastasis
- Endoplasmic Reticulum (ER) Stress and Vascular Complication