Yeungnam Univ J Med.
2020 Jul;37(3):230-235. 10.12701/yujm.2019.00423.
Impressive effect of cisplatin monotherapy on a patient with heavily pretreated triple-negative breast cancer with poor performance
- Affiliations
-
- 1Department of Oncology/Hematology, Kyungpook National University Chilgok Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
- 2Kyungpook National University Cancer Research Institute, Daegu, Korea
- 3Department of Pathology, Kyungpook National University Chilgok Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
- KMID: 2506523
- DOI: http://doi.org/10.12701/yujm.2019.00423
Abstract
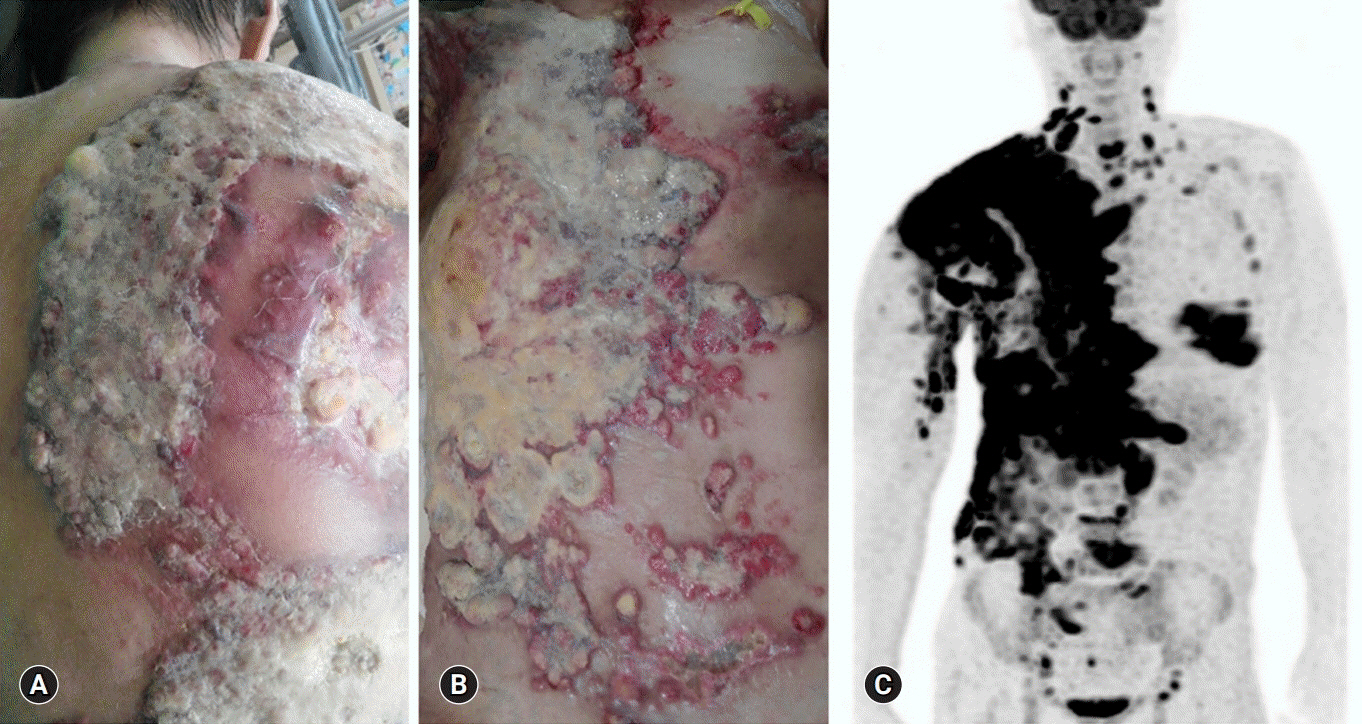
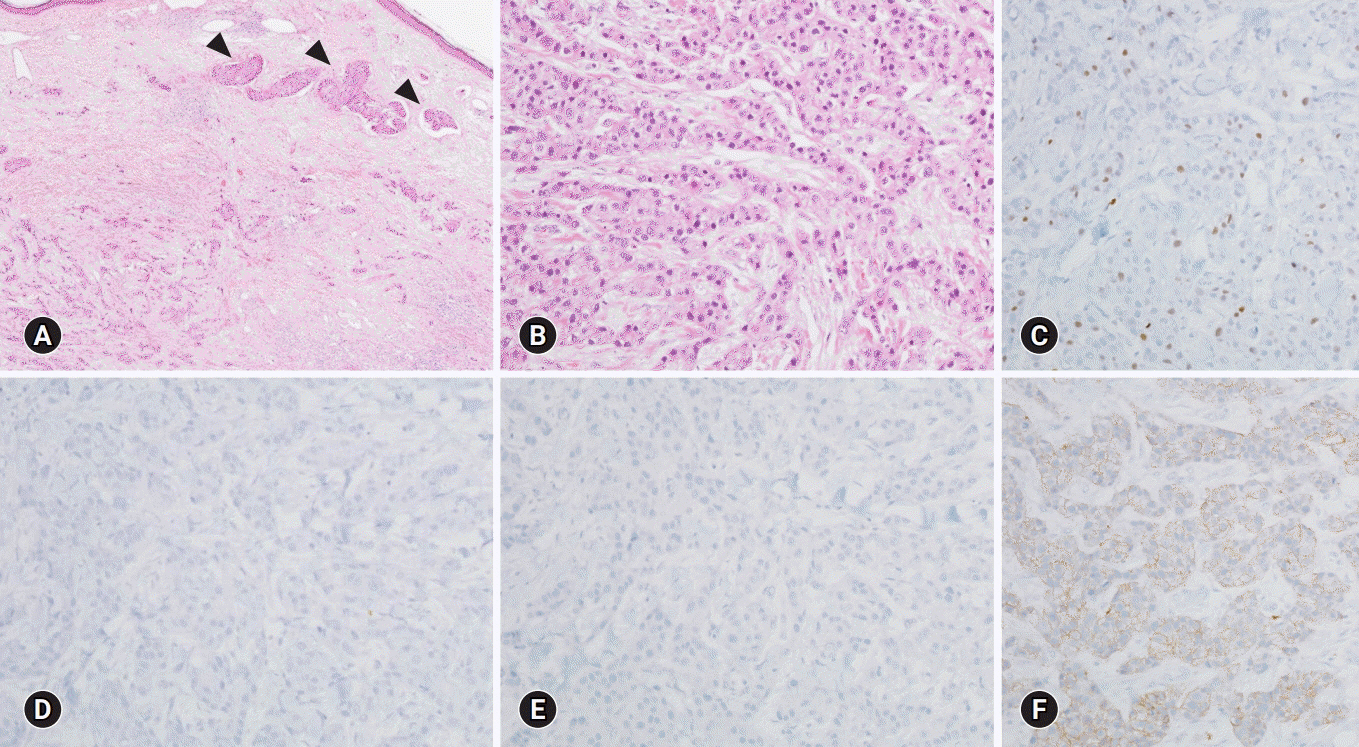
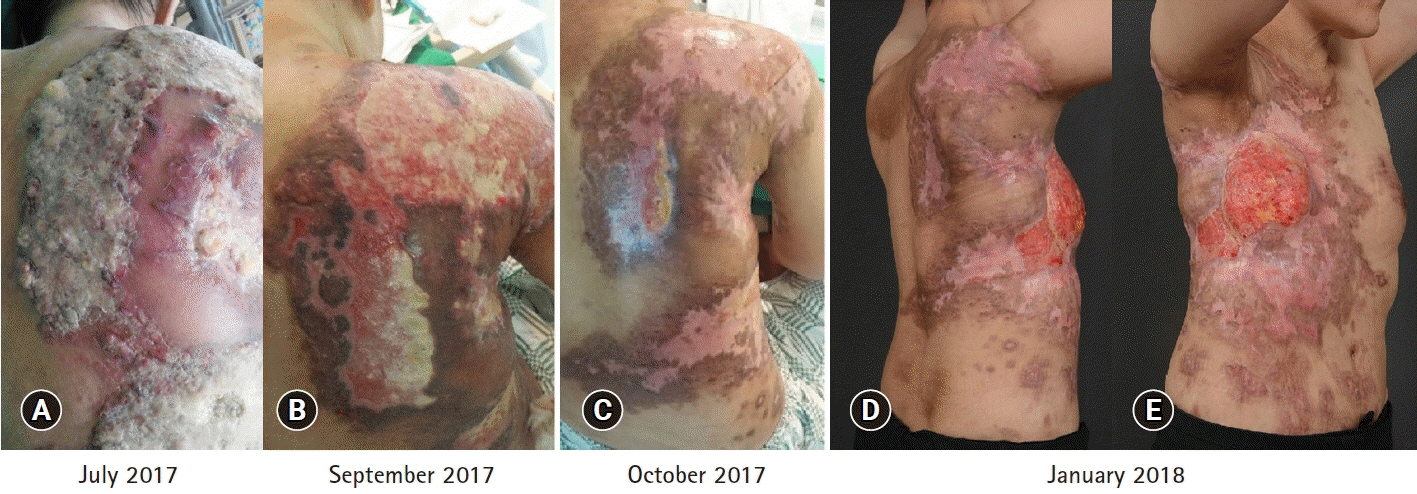
- Systemic therapy for metastatic triple-negative breast cancer (TNBC) still remains challenging because there are no targeted agents or endocrine therapies currently available. The present case report documents the successful use of cisplatin monotherapy to manage a heavily pretreated TNBC patient showing poor response to therapy. The patient was a 51-year-old woman who had already undergone several lines of systemic chemotherapy for widespread TNBC. Although the mutation analysis performed on DNA isolated from blood cells and progressed lesion samples confirmed the tumor to be germline BRCA wild-type, cisplatin monotherapy was administered based on the increasing evidence of safety and efficacy of platinum for breast cancer. After three cycles of cisplatin treatment, the patient’s metastatic lesions dramatically improved without any major toxicity, and she completed 17 cycles with good response. This case study indicates that patients with heavily pretreated TNBC can potentially achieve a good response to cisplatin monotherapy.
Figure
Reference
-
References
1. Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist. 2010; 15(Suppl 5):39–48.
Article2. O’Toole SA, Beith JM, Millar EK, West R, McLean A, Cazet A, et al. Therapeutic targets in triple negative breast cancer. J Clin Pathol. 2013; 66:530–42.
Article3. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007; 13:4429–34.
Article4. Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017; 377:523–33.
Article5. Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018; 379:753–63.
Article6. Mersin H, Yildirim E, Berberoglu U, Gulben K. The prognostic importance of triple negative breast carcinoma. Breast. 2008; 17:341–6.
Article7. National Comprehensive Cancer Network (NCCN). NCCN guidelines: breast cancer (version 3.2019) [Internet]. Plymouth Meeting (PA): NCCN;2019. Available at: https://www.nccn.org.8. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014; 384:164–72.
Article9. Florea AM, Busselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel). 2011; 3:1351–71.
Article10. Sirohi B, Arnedos M, Popat S, Ashley S, Nerurkar A, Walsh G, et al. Platinum-based chemotherapy in triple-negative breast cancer. Ann Oncol. 2008; 19:1847–52.
Article11. Koshy N, Quispe D, Shi R, Mansour R, Burton GV. Cisplatin-gemcitabine therapy in metastatic breast cancer: improved outcome in triple negative breast cancer patients compared to non-triple negative patients. Breast. 2010; 19:246–8.
Article12. Agrawal LS, Mayer IA. Platinum agents in the treatment of early-stage triple-negative breast cancer: is it time to change practice? Clin Adv Hematol Oncol. 2014; 12:654–8.13. Staudacher L, Cottu PH, Dieras V, Vincent-Salomon A, Guilhaume MN, Escalup L, et al. Platinum-based chemotherapy in metastatic triple-negative breast cancer: the Institut Curie experience. Ann Oncol. 2011; 22:848–56.
Article14. Isakoff SJ, Mayer EL, He L, Traina TA, Carey LA, Krag KJ, et al. TBCRC009: a multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol. 2015; 33:1902–9.
Article15. Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, et al. Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J Clin Oncol. 2010; 28:1145–53.
Article16. Cortesi L, Masini C, Cirilli C, Medici V, Marchi I, Cavazzini G, et al. Favourable ten-year overall survival in a Caucasian population with high probability of hereditary breast cancer. BMC Cancer. 2010; 10:90.
Article17. Zander SA, Kersbergen A, van der Burg E, de Water N, van Tellingen O, Gunnarsdottir S, et al. Sensitivity and acquired resistance of BRCA1;p53-deficient mouse mammary tumors to the topoisomerase I inhibitor topotecan. Cancer Res. 2010; 70:1700–10.
Article18. Hahnen E, Lederer B, Hauke J, Loibl S, Krober S, Schneeweiss A, et al. Germline mutation status, pathological complete response, and disease-free survival in triple negative breast cancer: secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncol. 2017; 3:1378–85.
Article19. Telli ML, Timms KM, Reid J, Hennessy B, Mills GB, Jensen KC, et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin Cancer Res. 2016; 22:3764–73.
Article20. Tutt A, Tovey H, Cheang MCU, Kernaghan S, Kilburn L, Gazinska P, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT trial. Nat Med. 2018; 24:628–37.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinicopathologic Characteristics and Prognosis of Early Stage Triple Negative Breast Cancer: Comparison with Non-triple Negative Group
- Comment to “Patients with Concordant Triple-Negative Phenotype between Primary Breast Cancers and Corresponding Metastases Have Poor Prognosisâ€
- Fear of Cancer Recurrence and Unmet Needs in Triple Negative Breast Cancer Survivors
- Treatment Outcomes of Weakly Positive Hormone Receptor Breast Cancer and Triple-Negative Breast Cancer
- Comment on “Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancerâ€