Obstet Gynecol Sci.
2020 Sep;63(5):675-678. 10.5468/ogs.19105.
Complications associated with intravesical migration of an intrauterine device
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Jahrom University of Medical Sciences, Jahrom, Iran
- 2Dr. Rasekh Clinic, Jahrom University of Medical Sciences, Jahrom, Iran
- KMID: 2506511
- DOI: http://doi.org/10.5468/ogs.19105
Abstract
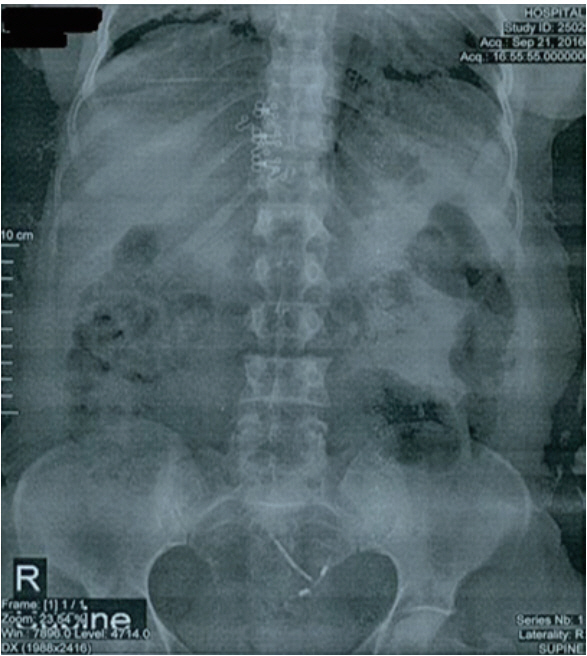
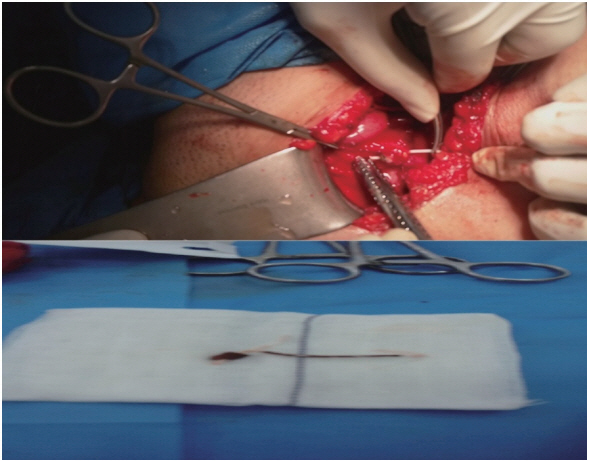
- The intrauterine device (IUD) is the most common method of reversible contraception in women. However, IUD can perforate the uterus and also migrate into pelvic or abdominal organs. A 43-year-old woman with a 5-year history of IUD placement and without specific symptoms, decided to remove her IUD and undergo tubal ligation. Radiological assessment, including a pelvic X-ray and ultrasonography, revealed no copper IUD within the uterus. Retrieval attempts with cystoscopy were unsuccessful. The IUD was found embedded in the fundal part of the bladder wall and was subsequently removed through a laparotomy incision. Although there are cases in the literature that were successfully managed with cystoscopy, in chronic cases, the formation of granulation tissue may preclude retrieval of an IUD using this intervention.
Keyword
Figure
Reference
-
References
1. Kaneshiro B, Aeby T. Long-term safety, efficacy, and patient acceptability of the intrauterine Copper T-380A contraceptive device. Int J Womens Health. 2010; 2:211–20.2. Istanbulluoglu MO, Ozcimen EE, Ozturk B, Uckuyu A, Cicek T, Gonen M. Bladder perforation related to intrauterine device. J Chin Med Assoc. 2008; 71:207–9.
Article3. Aghaways I, Anwer Wahid S, Ali RH, Sabir F, Kakamad FH. Migration of an intrauterine device to the left inguinal region, the first reported case. Int J Surg Case Rep. 2016; 28:68–70.
Article4. Faculty of Family Planning and Reproductive Health Care; Royal College of Obstetricians and Gynecologists. UK selected practice recommendations for contraceptive use. London: FFPRHC and RCOG;2003.5. Akpinar F, Ozgur EN, Yilmaz S, Ustaoglu O. Sigmoid colon migration of an intrauterine device. Case Rep Obstet Gynecol. 2014; 2014:207659.
Article6. Shin DG, Kim TN, Lee W. Intrauterine device embedded into the bladder wall with stone formation: laparoscopic removal is a minimally invasive alternative to open surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2012; 23:1129–31.
Article7. Tosun M, Celik H, Yavuz E, Çetinkaya MB. Intravesical migration of an intrauterine device detected in a pregnant woman. Can Urol Assoc J. 2010; 4:E141–3.
Article8. Sepúlveda WH, Ciuffardi I, Olivari A, Gallegos O. Sonographic diagnosis of bladder perforation by an intrauterine device. A case report. J Reprod Med. 1993; 38:911–3.9. Gyasi-Sarpong CK, Maison PO, Morhe E, Aboah K, Appiah KA, Azorliade R, et al. Intravesical migration of an intrauterine device. BMC Res Notes. 2016; 9:4.
Article10. Kaislasuo J, Suhonen S, Gissler M, Lähteenmäki P, Heikinheimo O. Uterine perforation caused by intrauterine devices: clinical course and treatment. Hum Reprod. 2013; 28:1546–51.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Intravesical Migration of Intrauterine Device with Stone Formation
- Intravesical Migration of an Intrauterine Contraceptive Device with Secondary Calculus Formation
- A case of cystolithiasis after intravesical migration of Copper T intrauterine device
- Laparoscopic Removal of an Intrauterine Contraceptive Device That Had Migrated into the Intraabdominal Cavity
- Bladder Stone Formation on Intra-uterine Contraceptive Device Perforated into the Bladder