Nutr Res Pract.
2020 Aug;14(4):309-321. 10.4162/nrp.2020.14.4.309.
Folic acid supplementation prevents high fructose-induced non-alcoholic fatty liver disease by activating the AMPK and LKB1 signaling pathways
- Affiliations
-
- 1Department of Food and Nutrition, College of Bio-Nano Science, Hannam University, Daejeon 34054, Korea
- KMID: 2504748
- DOI: http://doi.org/10.4162/nrp.2020.14.4.309
Abstract
- BACKGROUND/OBJECTIVES
The present study aimed to evaluate the effects of folic acid supplementation in high-fructose-induced hepatic steatosis and clarify the underlying mechanism of folic acid supplementation.
MATERIALS/METHODS
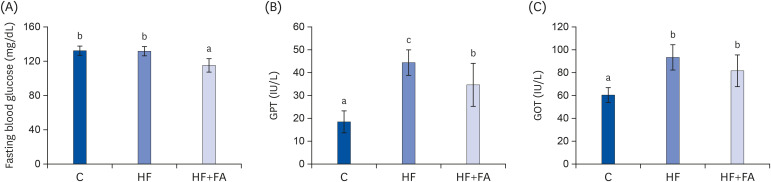
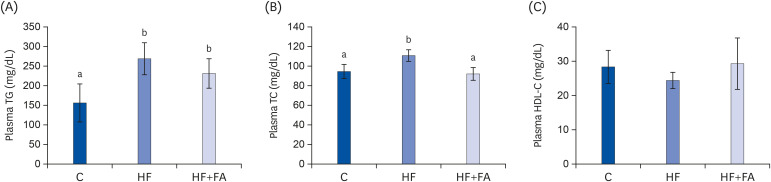
Male SD rats were fed control, 64% high-fructose diet, or 64% high-fructose diet with folic acid for eight weeks. Plasma glutamate-pyruvate transaminase, glutamate-oxaloacetate transaminase, lipid profiles, hepatic lipid content, S-adenosylmethionine (SAM), and S-adenosylhomocysteine (SAH) were measured.
RESULTS
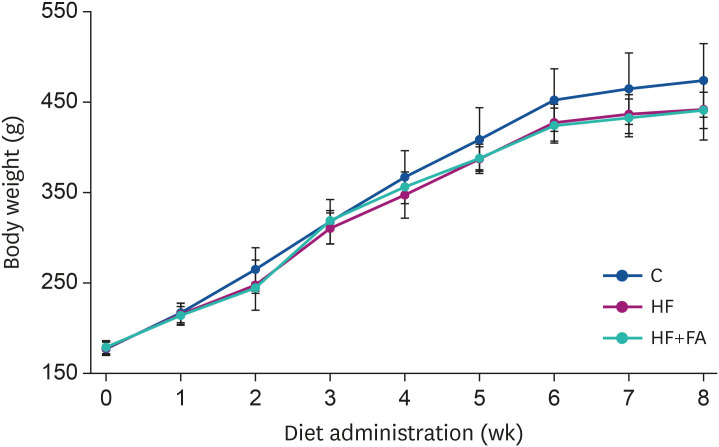
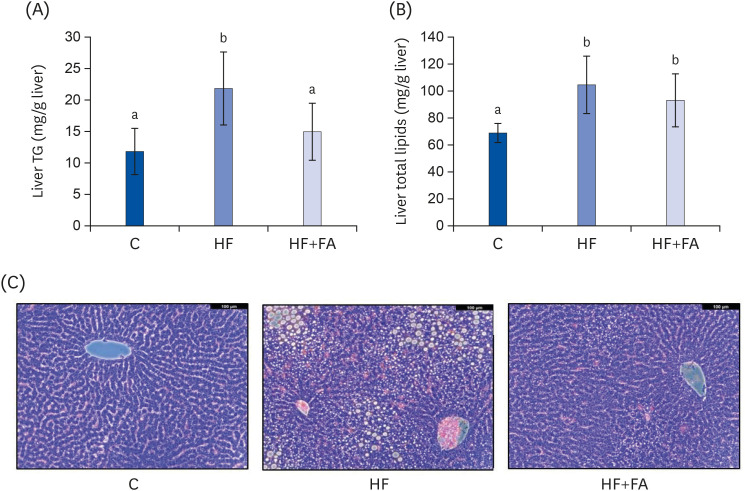
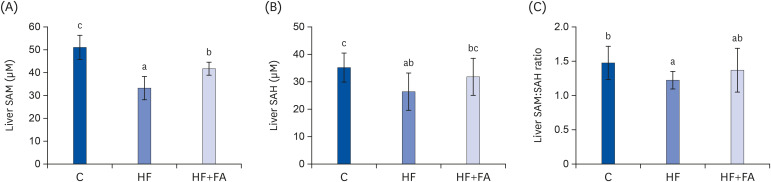
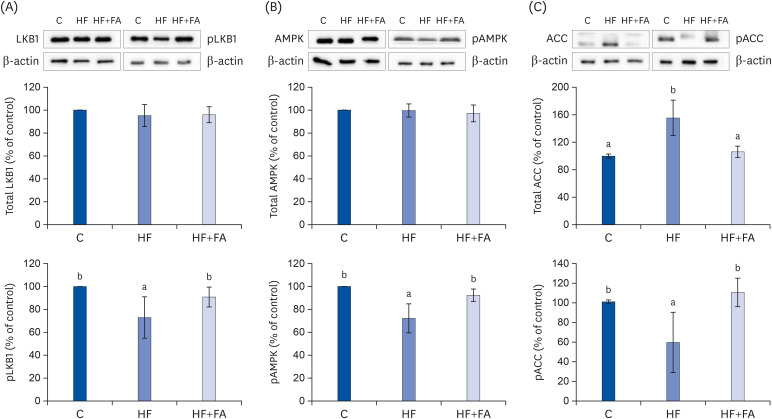
The HF diet significantly increased hepatic total lipid and triglyceride (TG) and decreased hepatic SAM, SAH, and SAM:SAH ratio. In rats fed a high fructose diet, folic acid supplementation significantly reduced hepatic TG, increased hepatic SAM, and alleviated hepatic steatosis. Moreover, folic acid supplementation in rats fed high fructose enhanced the levels of phosphorylated AMP-activated protein kinase (AMPK) and liver kinase B (LKB1) and inhibited phosphorylation of acetyl coenzyme A carboxylase (ACC) in the liver.
CONCLUSIONS
These results suggest that the protective effect of folic acid supplementation in rats fed high fructose may include the activation of LKB1/AMPK/ACC and increased SAM in the liver, which inhibit hepatic lipogenesis, thus ameliorating hepatic steatosis. The present study may provide evidence for the beneficial effects of folic acid supplementation in the treatment of non-alcoholic fatty liver disease.
Figure
Reference
-
1. Chitturi S, Wong VW, Farrell G. Nonalcoholic fatty liver in Asia: firmly entrenched and rapidly gaining ground. J Gastroenterol Hepatol. 2011; 26 Suppl 1:163–172. PMID: 21199528.
Article2. McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004; 8:521–533. PMID: 15331061.
Article3. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999; 94:2467–2474. PMID: 10484010.
Article4. Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010; 51:595–602. PMID: 20014114.
Article5. Dajani A, AbuHammour A. Treatment of nonalcoholic fatty liver disease: where do we stand? An overview. Saudi J Gastroenterol. 2016; 22:91–105. PMID: 26997214.6. Fakhoury-Sayegh N, Trak-Smayra V, Khazzaka A, Esseily F, Obeid O, Lahoud-Zouein M, Younes H. Characteristics of nonalcoholic fatty liver disease induced in Wistar rats following four different diets. Nutr Res Pract. 2015; 9:350–357. PMID: 26244072.
Article7. Kawasaki T, Igarashi K, Koeda T, Sugimoto K, Nakagawa K, Hayashi S, Yamaji R, Inui H, Fukusato T, Yamanouchi T. Rats fed fructose-enriched diets have characteristics of nonalcoholic hepatic steatosis. J Nutr. 2009; 139:2067–2071. PMID: 19776184.
Article8. Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. 1993; 58:754S–765S. PMID: 8213607.
Article9. Commerford SR, Ferniza JB, Bizeau ME, Thresher JS, Willis WT, Pagliassotti MJ. Diets enriched in sucrose or fat increase gluconeogenesis and G-6-Pase but not basal glucose production in rats. Am J Physiol Endocrinol Metab. 2002; 283:E545–55. PMID: 12169448.
Article10. Dirlewanger M, Schneiter P, Jéquier E, Tappy L. Effects of fructose on hepatic glucose metabolism in humans. Am J Physiol Endocrinol Metab. 2000; 279:E907–E911. PMID: 11001775.
Article11. Pagliassotti MJ, Wei Y, Bizeau ME. Glucose-6-phosphatase activity is not suppressed but the mRNA level is increased by a sucrose-enriched meal in rats. J Nutr. 2003; 133:32–37. PMID: 12514263.
Article12. Basciano H, Federico L, Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab (Lond). 2005; 2:5. PMID: 15723702.13. Ajiboye TO, Aliyu H, Tanimu MA, Muhammad RM, Ibitoye OB. Dioscoreophyllum cumminsii (Stapf) Diels leaves halt high-fructose induced metabolic syndrome: hyperglycemia, insulin resistance, inflammation and oxidative stress. J Ethnopharmacol. 2016; 192:471–479. PMID: 27568876.14. Robinson K, Mayer EL, Miller DP, Green R, van Lente F, Gupta A, Kottke-Marchant K, Savon SR, Selhub J, Nissen SE, Kutner M, Topol EJ, Jacobsen DW. Hyperhomocysteinemia and low pyridoxal phosphate. Common and independent reversible risk factors for coronary artery disease. Circulation. 1995; 92:2825–2830. PMID: 7586248.15. Mansoor MA, Svardal AM, Schneede J, Ueland PM. Dynamic relation between reduced, oxidized, and protein-bound homocysteine and other thiol components in plasma during methionine loading in healthy men. Clin Chem. 1992; 38:1316–1321. PMID: 1623597.
Article16. Suliman ME, Filho JC, Bárány P, Anderstam B, Lindholm B, Bergström J. Effects of methionine loading on plasma and erythrocyte sulphur amino acids and sulph-hydryls before and after co-factor supplementation in haemodialysis patients. Nephrol Dial Transplant. 2001; 16:102–110.
Article17. Noureddin M, Mato JM, Lu SC. Nonalcoholic fatty liver disease: update on pathogenesis, diagnosis, treatment and the role of S-adenosylmethionine. Exp Biol Med (Maywood). 2015; 240:809–820. PMID: 25873078.
Article18. Dahlhoff C, Worsch S, Sailer M, Hummel BA, Fiamoncini J, Uebel K, Obeid R, Scherling C, Geisel J, Bader BL, Daniel H. Methyl-donor supplementation in obese mice prevents the progression of NAFLD, activates AMPK and decreases acyl-carnitine levels. Mol Metab. 2014; 3:565–580. PMID: 25061561.
Article19. Cantó C, Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol Life Sci. 2010; 67:3407–3423. PMID: 20640476.
Article20. Woods A, Williams JR, Muckett PJ, Mayer FV, Liljevald M, Bohlooly-Y M, Carling D. Liver-specific activation of AMPK prevents steatosis on a high-fructose diet. Cell Reports. 2017; 18:3043–3051. PMID: 28355557.
Article21. Chang HC, Guarente L. Hung-ChunChang. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab. 2014; 25:138–145. PMID: 24388149.
Article22. Buettner R, Bettermann I, Hechtl C, Gäbele E, Hellerbrand C, Schölmerich J, Bollheimer LC. Dietary folic acid activates AMPK and improves insulin resistance and hepatic inflammation in dietary rodent models of the metabolic syndrome. Horm Metab Res. 2010; 42:769–774. PMID: 20803414.
Article23. Sid V, Wu N, Sarna LK, Siow YL, House JD, O K. Folic acid supplementation during high-fat diet feeding restores AMPK activation via an AMP-LKB1-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2015; 309:R1215–R1225. PMID: 26400185.
Article24. National Research Council (US) Subcommittee on Laboratory Animal Nutrition. Nutrient Requirements of Laboratory Animals: Fourth Revised Edition, 1995. Washington, D.C.: National Academies Press;1995.25. Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997; 127:838S–841S. PMID: 9164249.
Article26. Wagner J, Claverie N, Danzin C. A rapid high-performance liquid chromatographic procedure for the simultaneous determination of methionine, ethionine, S-adenosylmethionine, S-adenosylethionine, and the natural polyamines in rat tissues. Anal Biochem. 1984; 140:108–116. PMID: 6486398.
Article27. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509. PMID: 13428781.
Article28. Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007; 403:139–148. PMID: 17147517.
Article29. Mahesh M, Bharathi M, Reddy MR, Kumar MS, Putcha UK, Vajreswari A, Jeyakumar SM. Carrot juice administration decreases liver stearoyl-CoA desaturase 1 and improves docosahexaenoic acid levels, but not steatosis in high fructose diet-fed weanling Wistar rats. Prev Nutr Food Sci. 2016; 21:171–180. PMID: 27752492.
Article30. Bergheim I, Weber S, Vos M, Krämer S, Volynets V, Kaserouni S, McClain CJ, Bischoff SC. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008; 48:983–992. PMID: 18395289.
Article31. Weiß J, Rau M, Geier A. Non-alcoholic fatty liver disease: epidemiology, clinical course, investigation, and treatment. Dtsch Arztebl Int. 2014; 111:447–452. PMID: 25019921.32. Agren JJ, Kurvinen JP, Kuksis A. Isolation of very low density lipoprotein phospholipids enriched in ethanolamine phospholipids from rats injected with Triton WR 1339. Biochim Biophys Acta. 2005; 1734:34–43. PMID: 15866481.33. Jonas A, Phillips MC. Chapter 17. Lipoprotein structure. In : Vance DE, Vance JE, editors. Biochemistry of Lipids, Lipoproteins and Membranes. 5th ed. Boston: Elsevier Science;2008. p. 485–506.34. Cano A, Buqué X, Martínez-Uña M, Aurrekoetxea I, Menor A, García-Rodríguez JL, Lu SC, Martínez-Chantar ML, Mato JM, Ochoa B, Aspichueta P. Methionine adenosyltransferase 1A gene deletion disrupts hepatic very low-density lipoprotein assembly in mice. Hepatology. 2011; 54:1975–1986. PMID: 21837751.
Article35. Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans. 2002; 30:1064–1070. PMID: 12440973.
Article36. Hudson ER, Pan DA, James J, Lucocq JM, Hawley SA, Green KA, Baba O, Terashima T, Hardie DG. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Curr Biol. 2003; 13:861–866. PMID: 12747836.
Article37. Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009; 9:563–575. PMID: 19629071.
Article38. Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ, Witters LA, Kemp BE, Means AR. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 2008; 7:377–388. PMID: 18460329.
Article39. Tamás P, Hawley SA, Clarke RG, Mustard KJ, Green K, Hardie DG, Cantrell DA. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006; 203:1665–1670. PMID: 16818670.40. Stahmann N, Woods A, Carling D, Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase β. Mol Cell Biol. 2006; 26:5933–5945. PMID: 16880506.41. Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005; 2:9–19. PMID: 16054095.42. Ha J, Daniel S, Broyles SS, Kim KH. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J Biol Chem. 1994; 269:22162–22168. PMID: 7915280.
Article43. Wang Q, Liu S, Zhai A, Zhang B, Tian G. AMPK-mediated regulation of lipid metabolism by phosphorylation. Biol Pharm Bull. 2018; 41:985–993. PMID: 29709897.
Article44. Park SH, Gammon SR, Knippers JD, Paulsen SR, Rubink DS, Winder WW. Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J Appl Physiol (1985). 2002; 92:2475–2482. PMID: 12015362.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Folic acid supplementation reduces oxidative stress and hepatic toxicity in rats treated chronically with ethanol
- Understanding Bile Acid Signaling in Diabetes: From Pathophysiology to Therapeutic Targets
- Letter regarding “Auranofin attenuates hepatic steatosis and fibrosis in nonalcoholic fatty liver disease via NRF2 and NF-κB signaling pathways”
- PIK3R3 regulates PPARα expression to stimulate fatty acid β-oxidation and decrease hepatosteatosis
- Artemisia annua extract ameliorates high-fat diet-induced fatty liver by activating AMPK