Cancer Res Treat.
2020 Jul;52(3):764-778. 10.4143/crt.2020.044.
Clinical Implication of Concordant or Discordant Genomic Profiling between Primary and Matched Metastatic Tissues in Patients with Colorectal Cancer
- Affiliations
-
- 1Division of Oncology, Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 2Cancer Precision Medicine Diagnosis and Treatment Enterprise, Korea University Anam Hospital, Seoul, Korea
- 3School of Electrical Engineering, Korea University, Seoul, Korea
- 4Cancer Research Institute, Korea University, Seoul, Korea
- 5Department of Surgery, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 6Department of Thoracic Surgery, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- KMID: 2504458
- DOI: http://doi.org/10.4143/crt.2020.044
Abstract
- Purpose
The purpose of this study was to identify the concordant or discordant genomic profiling between primary and matched metastatic tumors in patients with colorectal cancer (CRC) and to explore the clinical implication.
Materials and Methods
Surgical samples of primary and matched metastatic tissues from 158 patients (335 samples) with CRC at Korea University Anam Hospital were evaluated using the Ion AmpliSeq Cancer Hotspot Panel. We compared genetic variants and classified them as concordant, primary-specific, and metastasis-specific variants. We used a combination of principal components analysis and clustering to find genomic groups. Kaplan-Meier curves were used to appraise survival between genomic groups. We used machine learning to confirm the correlation between genetic variants and metastatic sites.
Results
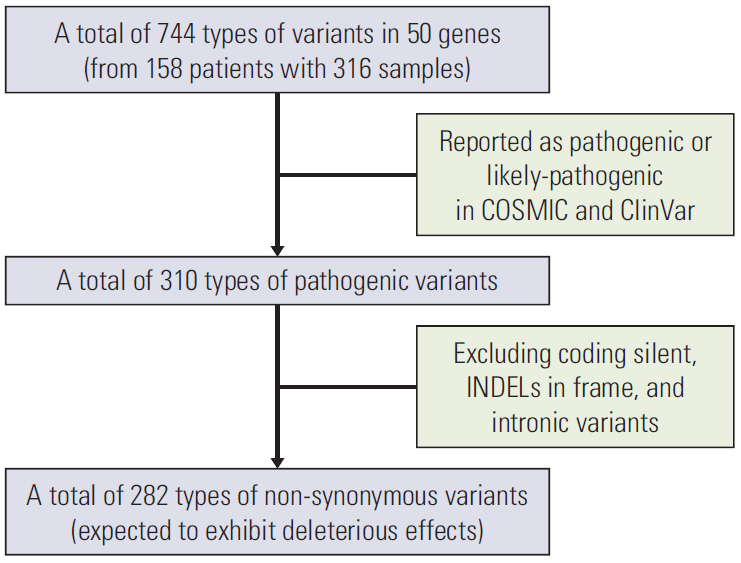
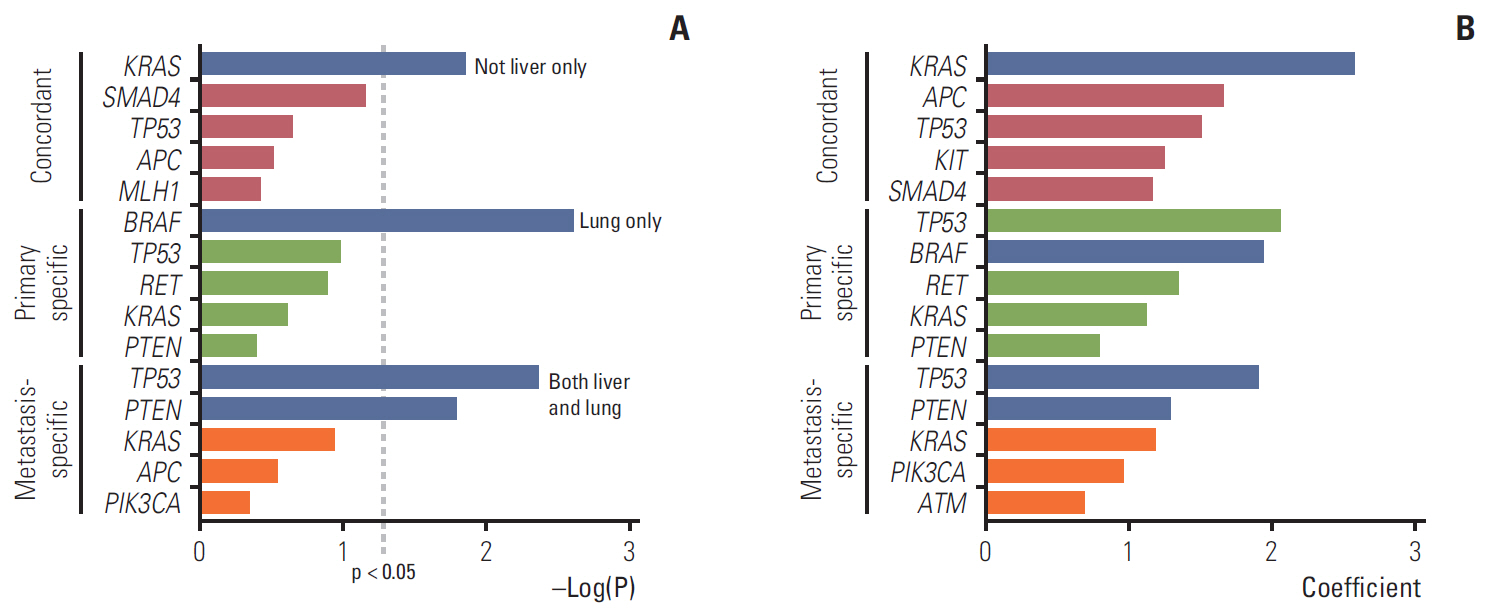
A total of 282 types of deleterious non-synonymous variants were selected for analysis. Of a total of 897 variants, an average of 40% was discordant. Three genomic groups were yielded based on the genomic discrepancy patterns. Overall survival differed significantly between the genomic groups. The poorest group had the highest proportion of concordant KRAS G12V and additional metastasis-specific SMAD4. Correlation analysis between genetic variants and metastatic sites suggested that concordant KRAS mutations would have more disseminated metastases.
Conclusion
Driver gene mutations were mostly concordant; however, discordant or metastasis-specific mutations were present. Clinically, the concordant driver genetic changes with additional metastasis-specific variants can predict poor prognosis for patients with CRC.
Figure
Cited by 1 articles
-
Clinical Application of Targeted Deep Sequencing in Metastatic Colorectal Cancer Patients: Actionable Genomic Alteration in K-MASTER Project
Youngwoo Lee, Soohyeon Lee, Jae Sook Sung, Hee-Joon Chung, Ah-reum Lim, Ju Won Kim, Yoon Ji Choi, Kyong Hwa Park, Yeul Hong Kim
Cancer Res Treat. 2021;53(1):123-130. doi: 10.4143/crt.2020.559.
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Riihimaki M, Thomsen H, Sundquist K, Hemminki K. Colorectal cancer patients: what do they die of? Frontline Gastroenterol. 2012; 3:143–9.
Article3. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995; 13:8–10.
Article4. Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011; 8:378–82.
Article5. Andres A, Mentha G, Adam R, Gerstel E, Skipenko OG, Barroso E, et al. Surgical management of patients with colorectal cancer and simultaneous liver and lung metastases. Br J Surg. 2015; 102:691–9.
Article6. de Baere T, Auperin A, Deschamps F, Chevallier P, Gaubert Y, Boige V, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol. 2015; 26:987–91.7. Massaut E, Bohlok A, Lucidi V, Hendlisz A, Klastersky JA, Donckier V. The concept of oligometastases in colorectal cancer: from the clinical evidences to new therapeutic strategies. Curr Opin Oncol. 2018; 30:262–8.
Article8. Blackmon SH, Stephens EH, Correa AM, Hofstetter W, Kim MP, Mehran RJ, et al. Predictors of recurrent pulmonary metastases and survival after pulmonary metastasectomy for olorectal cancer. Ann Thorac Surg. 2012; 94:1802–9.9. Lin J, Peng J, Zhao Y, Luo B, Zhao Y, Deng Y, et al. Early recurrence in patients undergoing curative resection of colorectal liver oligometastases: identification of its clinical characteristics, risk factors, and prognosis. J Cancer Res Clin Oncol. 2018; 144:359–69.
Article10. Schweiger T, Liebmann-Reindl S, Glueck O, Starlinger P, Laengle J, Birner P, et al. Mutational profile of colorectal cancer lung metastases and paired primary tumors by targeted next generation sequencing: implications on clinical outcome after surgery. J Thorac Dis. 2018; 10:6147–57.
Article11. Rehman AH, Jones RP, Poston G. Prognostic and predictive markers in liver limited stage IV colorectal cancer. Eur J Surg Oncol. 2019; 45:2251–6.
Article12. Pitroda SP, Khodarev NN, Huang L, Uppal A, Wightman SC, Ganai S, et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat Commun. 2018; 9:1793.
Article13. Bedard PL, Hansen AR, Ratain MJ, Siu LL. Tumour heterogeneity in the clinic. Nature. 2013; 501:355–64.
Article14. Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012; 366:883–92.
Article15. Pectasides E, Stachler MD, Derks S, Liu Y, Maron S, Islam M, et al. Genomic heterogeneity as a barrier to precision medicine in gastroesophageal adenocarcinoma. Cancer Discov. 2018; 8:37–48.
Article16. Zellmer VR, Zhang S. Evolving concepts of tumor heterogeneity. Cell Biosci. 2014; 4:69.
Article17. Kamps R, Brandao RD, Bosch BJ, Paulussen AD, Xanthoulea S, Blok MJ, et al. Next-generation sequencing in oncology: genetic diagnosis, risk prediction and cancer classification. Int J Mol Sci. 2017; 18:E308.
Article18. Huang D, Sun W, Zhou Y, Li P, Chen F, Chen H, et al. Mutations of key driver genes in colorectal cancer progression and metastasis. Cancer Metastasis Rev. 2018; 37:173–87.
Article19. Schlussel AT, Donlon SS, Eggerding FA, Gagliano RA. Identification of an APC variant in a patient with clinical attenuated familial adenomatous polyposis. Case Rep Med. 2014; 2014:432324.
Article20. Inra JA, Steyerberg EW, Grover S, McFarland A, Syngal S, Kastrinos F. Racial variation in frequency and phenotypes of APC and MUTYH mutations in 6,169 individuals undergoing genetic testing. Genet Med. 2015; 17:815–21.
Article21. Brannon AR, Vakiani E, Sylvester BE, Scott SN, McDermott G, Shah RH, et al. Comparative sequencing analysis reveals high genomic concordance between matched primary and metastatic colorectal cancer lesions. Genome Biol. 2014; 15:454.
Article22. Cross W, Graham TA, Wright NA. New paradigms in clonal evolution: punctuated equilibrium in cancer. J Pathol. 2016; 240:126–36.
Article23. Sottoriva A, Kang H, Ma Z, Graham TA, Salomon MP, Zhao J, et al. A Big Bang model of human colorectal tumor growth. Nat Genet. 2015; 47:209–16.
Article24. Barault L, Veyrie N, Jooste V, Lecorre D, Chapusot C, Ferraz JM, et al. Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH kinase) signaling network correlate with poor survival in a population-based series of colon cancers. Int J Cancer. 2008; 122:2255–9.25. Jones RP, Sutton PA, Evans JP, Clifford R, McAvoy A, Lewis J, et al. Specific mutations in KRAS codon 12 are associated with worse overall survival in patients with advanced and recurrent colorectal cancer. Br J Cancer. 2017; 116:923–9.26. Cai J, Xia L, Li J, Ni S, Song H, Wu X. Tumor-associated macrophages derived TGF-betaInduced epithelial to mesenchymal transition in colorectal cancer cells through Smad2,3-4/Snail signaling pathway. Cancer Res Treat. 2019; 51:252–66.27. Joung JG, Oh BY, Hong HK, Al-Khalidi H, Al-Alem F, Lee HO, et al. Tumor heterogeneity predicts metastatic potential in colorectal cancer. Clin Cancer Res. 2017; 23:7209–16.
Article28. Datta J, Smith JJ, Chatila WK, McAuliffe JC, Kandoth C, Vakiani E, et al. Coaltered Ras/B-raf and TP53 is associated with extremes of survivorship and distinct patterns of metastasis in patients with metastatic colorectal cancer. Clin Cancer Res. 2020; 26:1077–85.
Article29. Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003; 3:453–8.
Article30. Supek F, Minana B, Valcarcel J, Gabaldon T, Lehner B. Synonymous mutations frequently act as driver mutations in human cancers. Cell. 2014; 156:1324–35.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Genomic Profiling of Prostate Cancer: An Updated Review
- Patients with Concordant Triple-Negative Phenotype between Primary Breast Cancers and Corresponding Metastases Have Poor Prognosis
- Genomic Profiling of Liver Cancer
- Metastatic colon cancer of an ovarian cancer origin mimicking primary colon cancer: A case report
- Expression of Tumor Metastasis Related Genes in Korean Colorectal Cancers and Cell lines