J Rheum Dis.
2020 Jul;27(3):159-167. 10.4078/jrd.2020.27.3.159.
The Impact of the Amendment of the Korean National Health Insurance Reimbursement Criteria for Anti-tumor Necrosis Factor-α Agents on Treatment Pattern, Clinical Response and Persistence in Patients With Rheumatoid Arthritis
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea
- 2Division of Rheumatology, Department of Internal Medicine, Gyeongsang National University School of Medicine, Gyeongsang National University Changwon Hospital, Changwon, Korea
- 3Division of Rheumatology, Department of Internal Medicine, Pusan National University Hospital, Pusan National University School of Medicine, Korea
- 4Biomedical Research Institute, Pusan National University Hospital, Busan, Korea
- KMID: 2504031
- DOI: http://doi.org/10.4078/jrd.2020.27.3.159
Abstract
Objective
. To investigate the impact of the amendment of the Korean National Health Insurance (KNHI) reimbursement criteria for anti-tumor necrosis factor-α (TNF-α) agents based on from conventional clinical and laboratory measurements to disease activity score of 28 joints (DAS28) on treatment pattern, clinical response, and persistence rate in patients with rheumatoid arthritis (RA).
Methods
. This multicenter retrospective cohort study evaluated 148 RA patients eligible for the initiation of anti- TNF-α agents as the first-line biologics by either the past (n=95) or current (n=53) KNHI reimbursement criteria. Persistence was defined as the duration between the initiation and discontinuation of anti-TNFα agents.
Results
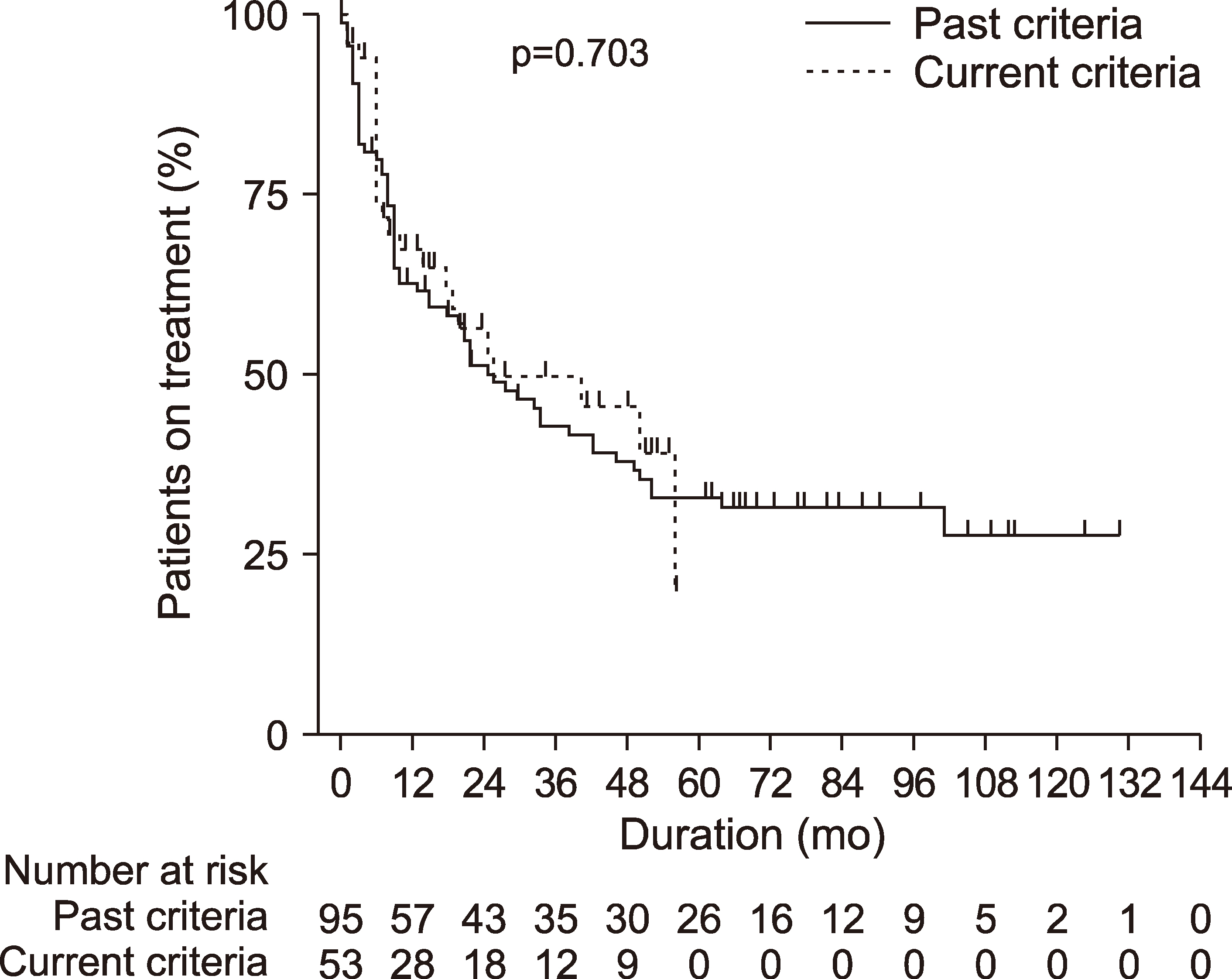
. In total, 106 (71.6%), 35 (23.6%), and 7 (4.7%) RA patients started treatment with adalimumab, etanercept, and infliximab, respectively. RA patients who received anti-TNF-α agents under the current reimbursement criteria had a significantly lower mean DAS28-erythrocyte sedimentation rate (ESR) (6.02 vs. 6.95, p<0.001) and daily prednisolone-equivalent glucocorticoid dose (4.51 vs. 6.17 mg, p<0.001) than those who received anti-TNF-α agents under the past reimbursement criteria. No significant differences in the 1-year remission rate defined by DAS28-ESR<2.6 (17.9% vs. 30.2%, p=0.085) and the persistence rate (p=0.703) between the past and current reimbursement criteria was observed.
Conclusion
. Our data suggest that less active RA patients can receive reimbursement for anti-TNF-α agents under the current criteria, and the amendment of the KNHI reimbursement criteria may improve access to anti-TNF-α agents without affecting the treatment response and persistence rate.
Keyword
Figure
Reference
-
1. Caporali R, Crepaldi G, Codullo V, Benaglio F, Monti S, Todoerti M, et al. 2018; 20 years of experience with tumour necrosis factor inhibitors: what have we learned? Rheumatology (Oxford). 57(57 Suppl 7):vii5–10. DOI: 10.1093/rheumatology/key059. PMID: 30289535.
Article2. Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2016; 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 68:1–26. DOI: 10.1002/art.39480. PMID: 26545940.
Article3. Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. 2017; EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 76:960–77. DOI: 10.1136/annrheumdis-2016-210715. PMID: 28264816.4. Souliotis K, Papageorgiou M, Politi A, Ioakeimidis D, Sidiropoulos P. 2014; Barriers to accessing biologic treatment for rheumatoid arthritis in Greece: the unseen impact of the fiscal crisis--the Health Outcomes Patient Environment (HOPE) study. Rheumatol Int. 34:25–33. DOI: 10.1007/s00296-013-2866-1. PMID: 24057144.
Article5. Desai RJ, Rao JK, Hansen RA, Fang G, Maciejewski ML, Farley JF. 2014; Predictors of treatment initiation with tumor necrosis factor-α inhibitors in patients with rheumatoid arthritis. J Manag Care Spec Pharm. 20:1110–20. DOI: 10.18553/jmcp.2014.20.11.1110. PMID: 25351972.6. Son KM, Jung DM, Kim YB, Han JS, Seo YI, Jung YO, et al. 2012; Comparison Korean National Health Insurance Reimbursement and other guidelines for TNF-α blocker in rheumatoid arthritis. J Rheum Dis. 19:334–40. DOI: 10.4078/jrd.2012.19.6.334.7. Won S, Sung YK, Cho SK, Choi CB, Koh EM, Kim SK, et al. 2014; Prediction for TNF inhibitor users in RA patients according to reimbursement criteria based on DAS28. J Rheum Dis. 21:64–73. DOI: 10.4078/jrd.2014.21.2.64.
Article8. Lee YJ. 2014; Usage of Anti-TNFα agents in patients with rheumatoid arthritis and Korean National Health Insurance reimbursement criteria. J Rheum Dis. 21:107–9. DOI: 10.4078/jrd.2014.21.3.107.9. Lee JH, Cho SK, Choi CB, Sung YK, Bae SC. 2011; Impact of change in reimbursement guideline of rheumatoid arthritis on the short term persistence of Tumor Necrosis Factor (TNF) blockers. J Rheum Dis. 18:283–7. DOI: 10.4078/jrd.2011.18.4.283.
Article10. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. 1988; The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31:315–24. DOI: 10.1002/art.1780310302. PMID: 3358796.
Article11. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010; 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 62:2569–81. DOI: 10.1002/art.27584. PMID: 20872595.12. Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. 1995; Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 38:44–8. DOI: 10.1002/art.1780380107. PMID: 7818570.
Article13. van Riel PL. 2014; The development of the disease activity score (DAS) and the disease activity score using 28 joint counts (DAS28). Clin Exp Rheumatol. 32(5 Suppl 85):S-65–74.14. van Gestel AM, Prevoo ML, van't Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. 1996; Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 39:34–40. DOI: 10.1002/art.1780390105. PMID: 8546736.
Article15. Lee HN, Kim YK, Kim GT, Ahn E, So MW, Sohn DH, et al. 2019; Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio as predictors of 12-week treatment response and drug persistence of anti-tumor necrosis factor-α agents in patients with rheumatoid arthritis: a retrospective chart review analysis. Rheumatol Int. 39:859–68. DOI: 10.1007/s00296-019-04276-x. PMID: 30874873.16. Park JH, Park EK, Koo DW, Lee S, Lee SH, Kim GT, et al. 2017; Compliance and persistence with oral bisphosphonates for the treatment of osteoporosis in female patients with rheumatoid arthritis. BMC Musculoskelet Disord. 18:152. DOI: 10.1186/s12891-017-1514-4. PMID: 28399834. PMCID: PMC5387221.
Article17. Lee SG, Park EK, Park JH, Kweon SM, Kim YK, Kim GT. 2018; Compliance and persistence with hydroxychloroquine in South Korean patients with systemic lupus erythematosus. Lupus. 27:753–61. DOI: 10.1177/0961203317742712. PMID: 29157178.
Article18. Orlewska E, Ancuta I, Anic B, Codrenau C, Damjanov N, Djukic P, et al. 2011; Access to biologic treatment for rheumatoid arthritis in Central and Eastern European (CEE) countries. Med Sci Monit. 17:SR1–13. DOI: 10.12659/MSM.881697. PMID: 21455121. PMCID: PMC3539513.
Article19. Hoebert JM, Mantel-Teeuwisse AK, van Dijk L, Bijlsma JW, Leufkens HG. 2012; Do rheumatoid arthritis patients have equal access to treatment with new medicines?: tumour necrosis factor-alpha inhibitors use in four European countries. Health Policy. 104:76–83. DOI: 10.1016/j.healthpol.2011.10.011. PMID: 22079753.
Article20. Putrik P, Ramiro S, Kvien TK, Sokka T, Uhlig T, Boonen A. 2014; Variations in criteria regulating treatment with reimbursed biologic DMARDs across European countries. Are differences related to country's wealth? Ann Rheum Dis. 73:2010–21. DOI: 10.1136/annrheumdis-2013-203819. PMID: 23940213.
Article21. Putrik P, Ramiro S, Kvien TK, Sokka T, Pavlova M, Uhlig T, et al. 2014; Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis. 73:198–206. DOI: 10.1136/annrheumdis-2012-202603. PMID: 23467636.
Article22. Kaló Z, Vokó Z, Östör A, Clifton-Brown E, Vasilescu R, Battersby A, et al. 2017; Patient access to reimbursed biological disease-modifying antirheumatic drugs in the European region. J Mark Access Health Policy. 5:1345580. DOI: 10.1080/20016689.2017.1345580. PMID: 28740623. PMCID: PMC5508389.
Article23. Bergstra SA, Branco JC, Vega-Morales D, Salomon-Escoto K, Govind N, Allaart CF, et al. 2018; Inequity in access to bDMARD care and how it influences disease outcomes across countries worldwide: results from the METEOR-registry. Ann Rheum Dis. 77:1413–20. DOI: 10.1136/annrheumdis-2018-213289. PMID: 29980576.
Article24. Hur JW, Choe JY, Kim DW, Kim HA, Kim SH, Kim WU, et al. 2015; Rheumatoid arthritis patients fulfilling Korean National Health Insurance reimbursement guidelines for anti-tumor necrosis factor-α treatment and comparison to other guidelines. Rheumatol Int. 35:1817–23. DOI: 10.1007/s00296-015-3353-7. PMID: 26342296.25. Smolen JS, Breedveld FC, Burmester GR, Bykerk V, Dougados M, Emery P, et al. 2016; Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 75:3–15. DOI: 10.1136/annrheumdis-2015-207524. PMID: 25969430. PMCID: PMC4717393.26. Neovius M, Arkema EV, Olsson H, Eriksson JK, Kristensen LE, Simard JF, et al. 2015; Drug survival on TNF inhibitors in patients with rheumatoid arthritis comparison of adalimumab, etanercept and infliximab. Ann Rheum Dis. 74:354–60. DOI: 10.1136/annrheumdis-2013-204128. PMID: 24285495. PMCID: PMC4316855.
Article27. Park EY, Lee SG, Park EK, Koo DW, Park JH, Kim GT, et al. 2018; Drug survival and the associated predictors in South Korean patients with rheumatoid arthritis receiving tacrolimus. Korean J Intern Med. 33:193–202. DOI: 10.3904/kjim.2015.385. PMID: 27048254. PMCID: PMC5768536.28. Park JA, Lee MY, Nam JH, Shin JY, Wood R, Holbrook T, et al. 2020; Real-world treatment persistence of non-tumor necrosis factor inhibitors versus tumor necrosis factor inhibitors among patients with rheumatoid arthritis in South Korea. Curr Med Res Opin. 36:343–51. DOI: 10.1080/03007995.2019.1688271. PMID: 31670976.
Article29. Kang JH, Park DJ, Lee JW, Lee KE, Wen L, Kim TJ, et al. 2014; Drug survival rates of tumor necrosis factor inhibitors in patients with rheumatoid arthritis and ankylosing spondylitis. J Korean Med Sci. 29:1205–11. DOI: 10.3346/jkms.2014.29.9.1205. PMID: 25246737. PMCID: PMC4168172.
Article30. Favalli EG, Pregnolato F, Biggioggero M, Becciolini A, Penatti AE, Marchesoni A, et al. 2016; Twelve-year retention rate of first-line tumor necrosis factor inhibitors in rheumatoid arthritis: real-life data from a local registry. Arthritis Care Res (Hoboken). 68:432–9. DOI: 10.1002/acr.22788. PMID: 26556048.
Article31. Malaviya AP, Ostör AJ. 2012; Drug adherence to biologic DMARDS with a special emphasis on the benefits of subcutaneous abatacept. Patient Prefer Adherence. 6:589–96. DOI: 10.2147/PPA.S23786. PMID: 22936845. PMCID: PMC3429155.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impact of Change in Reimbursement Guideline of Rheumatoid Arthritis on the Short Term Persistence of Tumor Necrosis Factor (TNF) Blockers
- Comparison Korean National Health Insurance Reimbursement and Other Guidelines for TNF-alpha Blocker in Rheumatoid Arthritis
- Usage of Anti-TNFalpha Agents in Patients with Rheumatoid Arthritis and Korean National Health Insurance Reimbursement Criteria
- Clinical Characteristics of Korean Rheumatoid Arthritis Patients with Indications for TNF-alpha Blocker
- Review of Tumor Necrosis Factor Inhibitors on Rheumatoid Arthritis