J Neurocrit Care.
2020 Jun;13(1):32-40. 10.18700/jnc.200002.
Robotically assisted transcranial Doppler with artificial intelligence for assessment of cerebral vasospasm after subarachnoid hemorrhage
- Affiliations
-
- 1Department of Anesthesiology, Critical Care and Pain Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
- 2Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
- 3Neurosurgical service, Beth Israel Deaconess Medical center, Harvard Medical School, Boston, MA, USA
- 4Department of Anesthesiology, Boston Medical Center, Boston University School of Medicine, Boston, MA, USA
- KMID: 2503383
- DOI: http://doi.org/10.18700/jnc.200002
Abstract
- Background
Transcranial Doppler (TCD) ultrasound is an essential tool for the detection of cerebral vasospasm after subarachnoid hemorrhage (SAH) but is limited by the availability of skilled operators. We examined the clinical feasibility and concordance of a robotically assisted TCD system with artificial intelligence with routine handheld TCD after SAH.
Methods
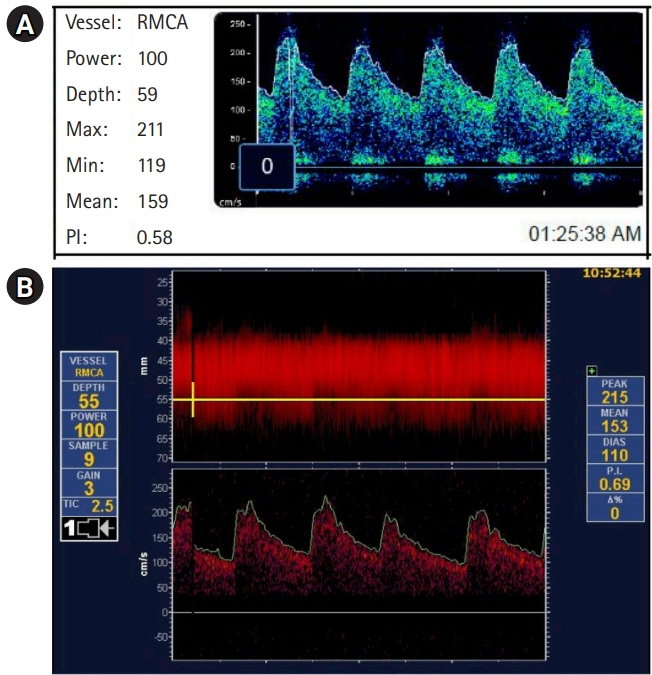
We evaluated TCD velocities in the anterior cerebral artery (ACA) and middle cerebral artery (MCA) of two patients with high-grade SAH and angiographic evidence of vasospasm. A single channel TCD device with a handheld diagnostic probe as well as a robotically assisted TCD device was used, the relationship of the two tests was evaluated using the bootstrap method of resampling for the concordance correlation coefficient (CCC) paired with a Pearson’s correlation analysis, followed by a Bland-Altman plot.
Results
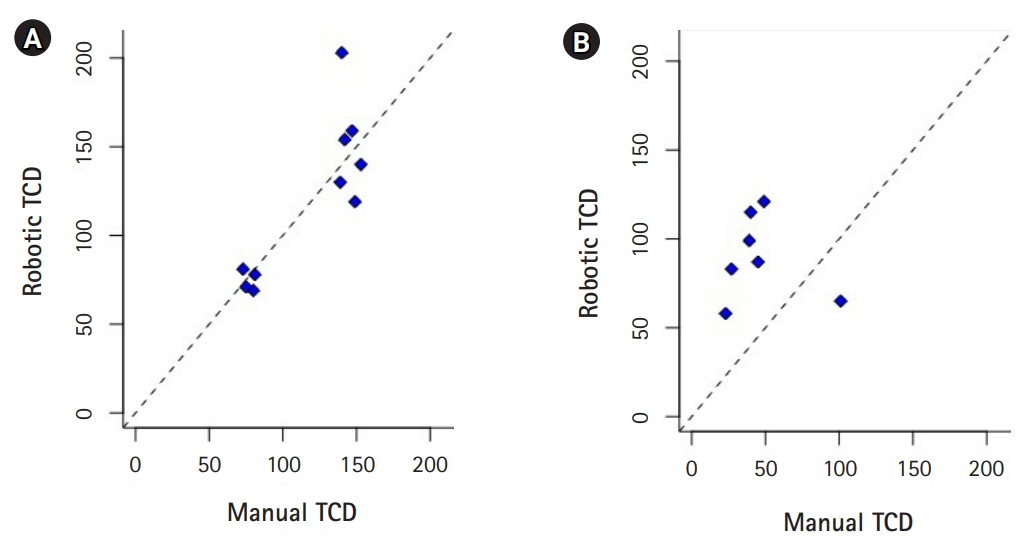
Patient 1 developed angiographic and TCD evidence of vasospasm in the proximal right MCA, but except for periods of disorientation remained neurologically intact. Angiographic, TCD and clinical evidence of ACA spasm occurred 6 days after ictus in patient 2. Robotically measured mean flow velocities were comparable to manual TCDs in the MCAs (CCC=0.83; 95% confidence interval [CI], 0.42 to 0.96; P=0.001) but not in the ACAs (CCC=0.26; 95% CI, –0.01 to 0.71; P=0.26).
Conclusion
Robotically assisted TCD system with artificial intelligence provides an alternative to manual TCD for assessment of MCA velocities in patients with SAH, expanding the availability of TCD to settings in which specialized clinicians are not available. Further studies for validation of this technology are warranted.
Keyword
Figure
Reference
-
1. Francoeur CL, Mayer SA. Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit Care. 2016; 20:277.
Article2. Kreiter KT, Copeland D, Bernardini GL, Bates JE, Peery S, Claassen J, et al. Predictors of cognitive dysfunction after subarachnoid hemorrhage. Stroke. 2002; 33:200–8.
Article3. Rowland MJ, Hadjipavlou G, Kelly M, Westbrook J, Pattinson KT. Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. Br J Anaesth. 2012; 109:315–29.
Article4. Schmid-Elsaesser R, Kunz M, Zausinger S, Prueckner S, Briegel J, Steiger HJ. Intravenous magnesium versus nimodipine in the treatment of patients with aneurysmal subarachnoid hemorrhage: a randomized study. Neurosurgery. 2006; 58:1054–65.
Article5. Daou BJ, Koduri S, Thompson BG, Chaudhary N, Pandey AS. Clinical and experimental aspects of aneurysmal subarachnoid hemorrhage. CNS Neurosci Ther. 2019; 25:1096–112.
Article6. Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012; 43:1711–37.7. Lannes M, Teitelbaum J, del Pilar Cortés M, Cardoso M, Angle M. Milrinone and homeostasis to treat cerebral vasospasm associated with subarachnoid hemorrhage: the Montreal Neurological Hospital protocol. Neurocrit Care. 2012; 16:354–62.
Article8. Sharma S, Lubrica RJ, Song M, Vandse R, Boling W, Pillai P. The role of transcranial Doppler in cerebral vasospasm: a literature review. Acta Neurochir Suppl. 2020; 127:201–5.
Article9. Schmidt JM, Wartenberg KE, Fernandez A, Claassen J, Rincon F, Ostapkovich ND, et al. Frequency and clinical impact of asymptomatic cerebral infarction due to vasospasm after subarachnoid hemorrhage. J Neurosurg. 2008; 109:1052–9.
Article10. Vora YY, Suarez-Almazor M, Steinke DE, Martin ML, Findlay JM. Role of transcranial Doppler monitoring in the diagnosis of cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 1999; 44:1237–48.
Article11. Grosset DG, Straiton J, du Trevou M, Bullock R. Prediction of symptomatic vasospasm after subarachnoid hemorrhage by rapidly increasing transcranial Doppler velocity and cerebral blood flow changes. Stroke. 1992; 23:674–9.
Article12. O’Brien M, Ranjbaran M, Ilyas P, Scheidt M, Thorpe S, Nicolas C, et al. Fully automated transcranial doppler ultrasound insonation of the MCA using a five degree of freedom robotically actuated probe system. Eur J Neurol. 2018; 25:56–57.13. Zeiler FA, Smielewski P. Application of robotic transcranial Doppler for extended duration recording in moderate/severe traumatic brain injury: first experiences. Crit Ultrasound J. 2018; 10:16.
Article14. Williamson JM, Crawford SB, Lin HM. Resampling dependent concordance correlation coefficients. J Biopharm Stat. 2007; 17:685–96.
Article15. Altman DG. Practical statistics for medical research. 1st ed. Boca Raton: CRC press;1990.16. Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007; 17:571–82.
Article17. Lehnert B. BlandAltmanLeh: Plots (Slightly Extended) Bland-Altman Plots. R package version 0312015. In: Cran R-project [Internet]. Vienna (AU): R Foundation;2015. [cited 2020 May 23]. Available from: https://CRAN.R-project.org/package=BlandAltmanLeh.18. Stevenson M, Heuer C, Marshall J, Sanchez J, Reiczigel J, Robison-Cox J, et al. epiR: Tools for the Analysis of Epidemiological Data. R package version 10-102019. In: Cran R-project [Internet]. Vienna (AU): R Foundation;2019. [cited 2020 May 23]. Available from: https://CRAN.R-project.org/package=epiR.19. R Core Team. R: A Language and Environment for Statistical Computing. In: R Foundation for Statistical Computing [Internet]. Vienna (AU): R Foundation;2018. [cited 2020 May 23]. Available from: https://www.R-project.org.20. Sekhar LN, Wechsler LR, Yonas H, Luyckx K, Obrist W. Value of transcranial Doppler examination in the diagnosis of cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 1988; 22:813–21.
Article21. Mizuno M, Nakajima S, Sampei T, Nishimura H, Hadeishi H, Suzuki A, et al. Serial transcranial Doppler flow velocity and cerebral blood flow measurements for evaluation of cerebral vasospasm after subarachnoid hemorrhage. Neurol Med Chir (Tokyo). 1994; 34:164–71.
Article22. Sloan MA, Haley EC Jr, Kassell NF, Henry ML, Stewart SR, Beskin RR, et al. Sensitivity and specificity of transcranial Doppler ultrasonography in the diagnosis of vasospasm following subarachnoid hemorrhage. Neurology. 1989; 39:1514–8.
Article23. Suarez JI, Qureshi AI, Yahia AB, Parekh PD, Tamargo RJ, Williams MA, et al. Symptomatic vasospasm diagnosis after subarachnoid hemorrhage: evaluation of transcranial Doppler ultrasound and cerebral angiography as related to compromised vascular distribution. Crit Care Med. 2002; 30:1348–55.
Article24. Seidel G, Kaps M, Gerriets T. Potential and limitations of transcranial color-coded sonography in stroke patients. Stroke. 1995; 26:2061–6.
Article25. Clark JM, Skolnick BE, Gelfand R, Farber RE, Stierheim M, Stevens WC, et al. Relationship of 133Xe cerebral blood flow to middle cerebral arterial flow velocity in men at rest. J Cereb Blood Flow Metab. 1996; 16:1255–62.
Article26. Aries MJ, Elting JW, De Keyser J, Kremer BP, Vroomen PC. Cerebral autoregulation in stroke: a review of transcranial Doppler studies. Stroke. 2010; 41:2697–704.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transcranial Doppler Monitoring in Subarachnoid Hemorrhage
- Transcranial Doppler Measurement of Intracraial Arterial Flow Velocity in Subarachnoid Hemorrhage
- Diagnostic significance of transcranial Doppler ultrasonography in patients with subarachnoid hemorrhage and cerebral vasospasm
- Is Transcranial Doppler Ultrasonography Old-fashioned?: One Institutional Validity Study
- Cerebral Vasospasm After Traumatic Subarachnoid Hemorrhage and Its Risk Factor: Combined Periodic Follow Up of Transcranial Doppler and CT Angiography