J Korean Med Sci.
2020 Jun;35(23):e188. 10.3346/jkms.2020.35.e188.
Evaluation of Human MSCs Treatment Frequency on Airway Inflammation in a Mouse Model of Acute Asthma
- Affiliations
-
- 1Division of Allergy and Pulmonary Medicine, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Hospital Pathology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2502923
- DOI: http://doi.org/10.3346/jkms.2020.35.e188
Abstract
- Background
Studies in experimental models of allergic asthma have shown that mesenchymal stem cells (MSCs) have therapeutic potential for T-helper 2 (TH2) cell-mediated inflammation. However, the mechanisms underlying these therapeutic effects are not fully understood and their safety has not been confirmed.
Methods
Using a mouse model of experimental allergic asthma, we investigated the efficacy of human adipose-derived mesenchymal stem cells (hADSCs) or human bone marrow-derived mesenchymal stem cells (hBMSCs) according to treatment frequency and timing.
Results
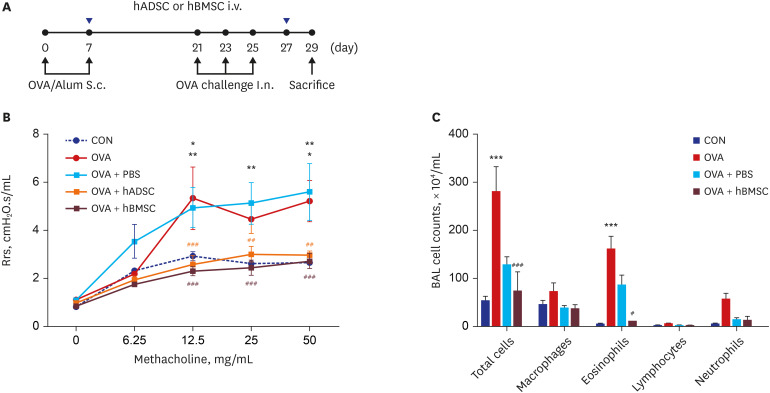
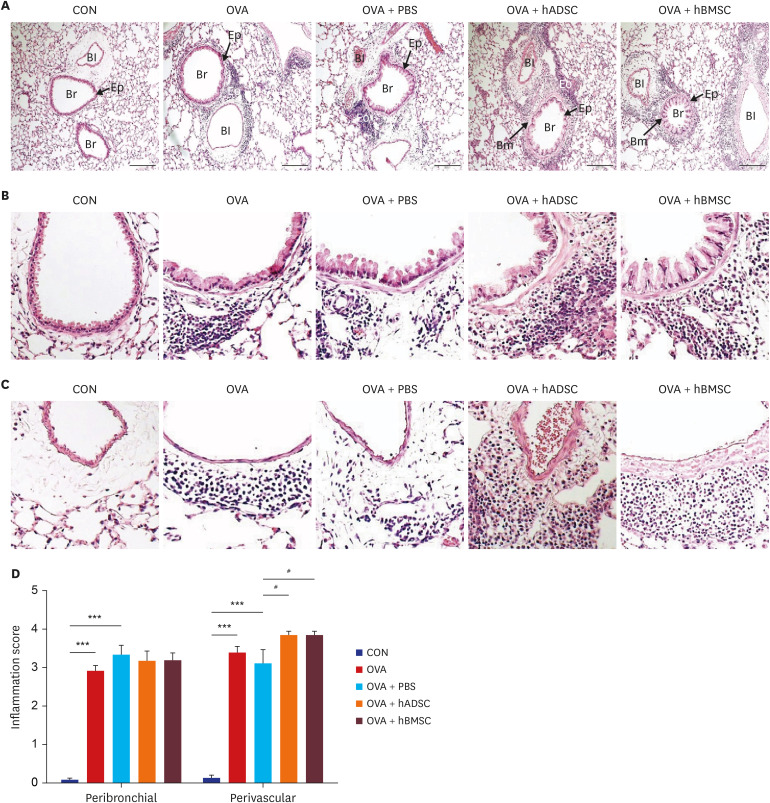
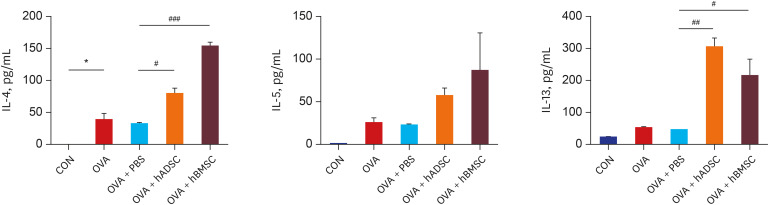
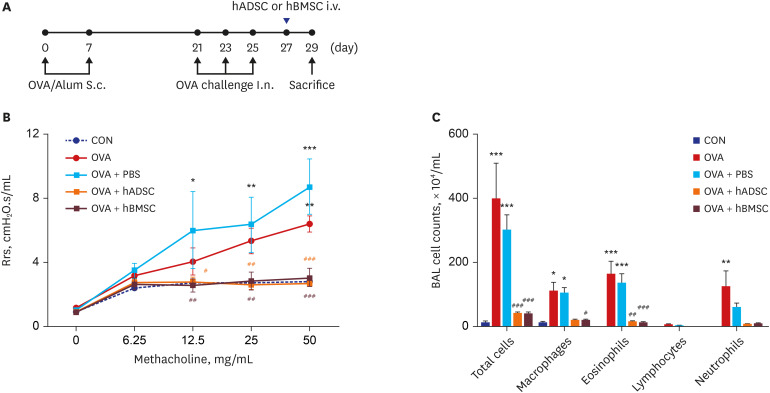
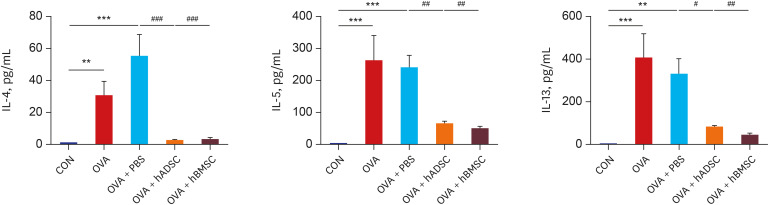
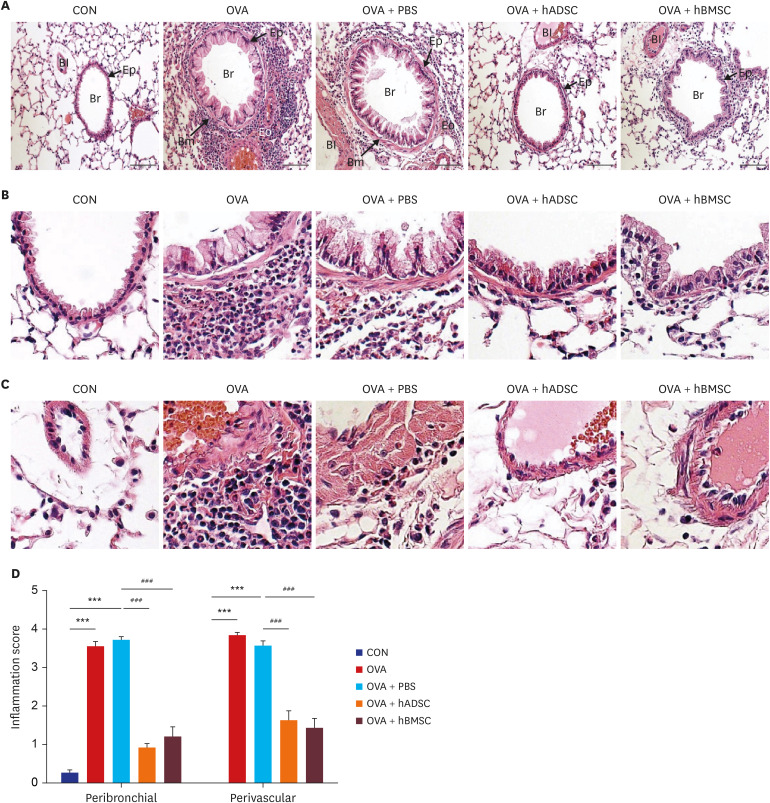
Ovalbumin (OVA)-sensitized and -challenged mice exhibited airway hyperresponsiveness (AHR), airway inflammation, and significant increases in TH2 cytokine levels. Both double and single human mesenchymal stem cell (hMSC) treatments significantly decreased AHR and bronchoalveolar lavage fluid counts. In addition, single treatment with hMSCs showed significant attenuation of allergic airway inflammation. However, double treatment with hMSCs during OVA -sensitization and -challenge further increased inflammatory cell infiltration, and TH2 cytokine levels.
Conclusion
The results of treatment with hADSCs or hBMSCs suppresses AHR and airway inflammation. However, double hMSC treatment significantly induces eosinophilic airway inflammation and lung histological changes. Therefore, double hMSC treatment is ineffective against asthma and single injection frequency appears to be more important for the treatment of asthma. These results suggest that hMSC therapy can be used for treatment of asthma patients but that it should be used carefully.
Keyword
Figure
Reference
-
1. Olin JT, Wechsler ME. Asthma: pathogenesis and novel drugs for treatment. BMJ. 2014; 349:g5517. PMID: 25420994.
Article2. Holgate ST, Arshad HS, Roberts GC, Howarth PH, Thurner P, Davies DE. A new look at the pathogenesis of asthma. Clin Sci (Lond). 2009; 118(7):439–450. PMID: 20025610.
Article3. Saglani S, Lloyd CM. Novel concepts in airway inflammation and remodelling in asthma. Eur Respir J. 2015; 46(6):1796–1804. PMID: 26541520.
Article4. Barnes PJ. Current therapies for asthma. Promise and limitations. Chest. 1997; 111(2):Suppl. 17S–26S. PMID: 9042023.5. Riccioni G, Di Ilio C, D'Orazio N. Review: Pharmacological treatment of airway remodeling: inhaled corticosteroids or antileukotrienes? Ann Clin Lab Sci. 2004; 34(2):138–142. PMID: 15228224.6. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007; 110(10):3499–3506. PMID: 17664353.
Article7. Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007; 262(5):509–525. PMID: 17949362.
Article8. Rasmusson I. Immune modulation by mesenchymal stem cells. Exp Cell Res. 2006; 312(12):2169–2179. PMID: 16631737.
Article9. Nejad-Moghaddam A, Panahi Y, Abdollahpour Alitappeh M, Borna H, Shokrgozar MA, Ghanei M. Therapeutic potential of mesenchymal stem cells for the treatment of airway remodeling in pulmonary diseases. Iran J Allergy Asthma Immunol. 2015; 14(6):552–568. PMID: 26725553.10. Kanelidis A, Premer C, Lopez JG, Balkan W, Hare JM. Route of delivery modulates the efficacy of mesenchymal stem cell therapy for myocardial infarction: a meta-analysis of preclinical studies and clinical trials. Circ Res. 2017; 120(7):1139–1150. PMID: 28031416.11. Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, Bagheri M, Bashtar M, Ghanaati H, et al. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007; 10(4):459–466. PMID: 17903050.12. Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004; 363(9419):1439–1441. PMID: 15121408.
Article13. Braza F, Dirou S, Forest V, Sauzeau V, Hassoun D, Chesné J, et al. Mesenchymal stem cells induce suppressive macrophages through phagocytosis in a mouse model of asthma. Stem Cells. 2016; 34(7):1836–1845. PMID: 26891455.
Article14. Mathias LJ, Khong SM, Spyroglou L, Payne NL, Siatskas C, Thorburn AN, et al. Alveolar macrophages are critical for the inhibition of allergic asthma by mesenchymal stromal cells. J Immunol. 2013; 191(12):5914–5924. PMID: 24249728.
Article15. Lathrop MJ, Brooks EM, Bonenfant NR, Sokocevic D, Borg ZD, Goodwin M, et al. Mesenchymal stromal cells mediate Aspergillus hyphal extract-induced allergic airway inflammation by inhibition of the Th17 signaling pathway. Stem Cells Transl Med. 2014; 3(2):194–205. PMID: 24436442.16. Goodwin M, Sueblinvong V, Eisenhauer P, Ziats NP, LeClair L, Poynter ME, et al. Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells. 2011; 29(7):1137–1148. PMID: 21544902.
Article17. Firinci F, Karaman M, Baran Y, Bagriyanik A, Ayyildiz ZA, Kiray M, et al. Mesenchymal stem cells ameliorate the histopathological changes in a murine model of chronic asthma. Int Immunopharmacol. 2011; 11(8):1120–1126. PMID: 21439399.
Article18. Gao P, Zhou Y, Xian L, Li C, Xu T, Plunkett B, et al. Functional effects of TGF-β1 on mesenchymal stem cell mobilization in cockroach allergen-induced asthma. J Immunol. 2014; 192(10):4560–4570. PMID: 24711618.
Article19. Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci U S A. 2010; 107(12):5652–5657. PMID: 20231466.20. Cho KS, Park MK, Kang SA, Park HY, Hong SL, Park HK, et al. Adipose-derived stem cells ameliorate allergic airway inflammation by inducing regulatory T cells in a mouse model of asthma. Mediators Inflamm. 2014; 2014:436476. PMID: 25246732.
Article21. Ogulur I, Gurhan G, Aksoy A, Duruksu G, Inci C, Filinte D, et al. Suppressive effect of compact bone-derived mesenchymal stem cells on chronic airway remodeling in murine model of asthma. Int Immunopharmacol. 2014; 20(1):101–109. PMID: 24613203.
Article22. Bonfield TL, Koloze M, Lennon DP, Zuchowski B, Yang SE, Caplan AI. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am J Physiol Lung Cell Mol Physiol. 2010; 299(6):L760–70. PMID: 20817776.
Article23. Hur J, Kang JY, Rhee CK, Kim YK, Lee SY. The leukotriene receptor antagonist pranlukast attenuates airway remodeling by suppressing TGF-β signaling. Pulm Pharmacol Ther. 2018; 48:5–14. PMID: 29031615.
Article24. Yoon DS, Kim YH, Lee S, Lee KM, Park KH, Jang Y, et al. Interleukin-6 induces the lineage commitment of bone marrow-derived mesenchymal multipotent cells through down-regulation of Sox2 by osteogenic transcription factors. FASEB J. 2014; 28(7):3273–3286. PMID: 24719354.
Article25. Finkelman FD, Hogan SP, Hershey GK, Rothenberg ME, Wills-Karp M. Importance of cytokines in murine allergic airway disease and human asthma. J Immunol. 2010; 184(4):1663–1674. PMID: 20130218.
Article26. Lee SH, Jang AS, Kwon JH, Park SK, Won JH, Park CS. Mesenchymal stem cell transfer suppresses airway remodeling in a toluene diisocyanate-induced murine asthma model. Allergy Asthma Immunol Res. 2011; 3(3):205–211. PMID: 21738887.
Article27. Ou-Yang HF, Huang Y, Hu XB, Wu CG. Suppression of allergic airway inflammation in a mouse model of asthma by exogenous mesenchymal stem cells. Exp Biol Med (Maywood). 2011; 236(12):1461–1467. PMID: 22114062.
Article28. Abreu SC, Antunes MA, Xisto DG, Cruz FF, Branco VC, Bandeira E, et al. Bone marrow, adipose, and lung tissue-derived murine mesenchymal stromal cells release different mediators and differentially affect airway and lung parenchyma in experimental asthma. Stem Cells Transl Med. 2017; 6(6):1557–1567. PMID: 28425576.
Article29. Kavanagh H, Mahon BP. Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy. 2011; 66(4):523–531. PMID: 21091718.
Article30. Sun YQ, Deng MX, He J, Zeng QX, Wen W, Wong DS, et al. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells. 2012; 30(12):2692–2699. PMID: 22987325.
Article31. Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012; 10(6):709–716. PMID: 22704511.
Article32. Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012; 12(5):383–396. PMID: 22531326.
Article33. Prockop DJ, Oh JY. Medical therapies with adult stem/progenitor cells (MSCs): a backward journey from dramatic results in vivo to the cellular and molecular explanations. J Cell Biochem. 2012; 113(5):1460–1469. PMID: 22213121.
Article34. Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013; 13(4):392–402. PMID: 24094322.
Article35. Bai L, Lennon DP, Eaton V, Maier K, Caplan AI, Miller SD, et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009; 57(11):1192–1203. PMID: 19191336.
Article36. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005; 105(4):1815–1822. PMID: 15494428.
Article37. Kang WC, Oh PC, Lee K, Ahn T, Byun K. Increasing injection frequency enhances the survival of injected bone marrow derived mesenchymal stem cells in a critical limb ischemia animal model. Korean J Physiol Pharmacol. 2016; 20(6):657–667. PMID: 27847443.
Article38. Swedin L, Neimert-Andersson T, Hjoberg J, Jonasson S, van Hage M, Adner M, et al. Dissociation of airway inflammation and hyperresponsiveness by cyclooxygenase inhibition in allergen challenged mice. Eur Respir J. 2009; 34(1):200–208. PMID: 19251789.
Article39. Wilder JA, Collie DD, Wilson BS, Bice DE, Lyons CR, Lipscomb MF. Dissociation of airway hyperresponsiveness from immunoglobulin E and airway eosinophilia in a murine model of allergic asthma. Am J Respir Cell Mol Biol. 1999; 20(6):1326–1334. PMID: 10340953.
Article40. Sharkhuu T, Matthaei KI, Forbes E, Mahalingam S, Hogan SP, Hansbro PM, et al. Mechanism of interleukin-25 (IL-17E)-induced pulmonary inflammation and airways hyper-reactivity. Clin Exp Allergy. 2006; 36(12):1575–1583. PMID: 17177681.
Article41. Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008; 20(6):791–800. PMID: 18448455.
Article42. Zhou B, Headley MB, Aye T, Tocker J, Comeau MR, Ziegler SF. Reversal of thymic stromal lymphopoietin-induced airway inflammation through inhibition of Th2 responses. J Immunol. 2008; 181(9):6557–6562. PMID: 18941246.
Article43. Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, et al. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007; 120(6):1324–1331. PMID: 17889290.
Article44. Onari Y, Yokoyama A, Haruta Y, Nakashima T, Iwamoto H, Hattori N, et al. IL-12p40 is essential for the down-regulation of airway hyperresponsiveness in a mouse model of bronchial asthma with prolonged antigen exposure. Clin Exp Allergy. 2009; 39(2):290–298. PMID: 19032358.
Article45. Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007; 39(2):573–576. PMID: 17362785.
Article46. Kang WJ, Kang HJ, Kim HS, Chung JK, Lee MC, Lee DS. Tissue distribution of 18F-FDG-labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction. J Nucl Med. 2006; 47(8):1295–1301. PMID: 16883008.47. Ge J, Guo L, Wang S, Zhang Y, Cai T, Zhao RC, et al. The size of mesenchymal stem cells is a significant cause of vascular obstructions and stroke. Stem Cell Rev Rep. 2014; 10(2):295–303. PMID: 24390934.
Article48. Bartosh TJ, Ylöstalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010; 107(31):13724–13729. PMID: 20643923.
Article49. Häfeli UO, Saatchi K, Elischer P, Misri R, Bokharaei M, Labiris NR, et al. Lung perfusion imaging with monosized biodegradable microspheres. Biomacromolecules. 2010; 11(3):561–567. PMID: 20143805.
Article50. Anjos-Afonso F, Siapati EK, Bonnet D. In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. J Cell Sci. 2004; 117(Pt 23):5655–5664. PMID: 15494370.
Article51. Lee RH, Seo MJ, Pulin AA, Gregory CA, Ylostalo J, Prockop DJ. The CD34-like protein PODXL and alpha6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood. 2009; 113(4):816–826. PMID: 18818395.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Understanding asthma using animal models
- Evolution of Asthma Concept and Effect of Current Asthma Management Guidelines
- Effect of DHEA on airway hyperresponsiveness and inflammation in murine model of asthma
- Understanding the Mouse Model of Respiratory Allergic Diseases
- Effects of Korean Red Ginseng Extracts on Airway Hyperresponsiveness and Inflammation in a Murine Asthma Model