Diabetes Metab J.
2020 Apr;44(2):326-335. 10.4093/dmj.2019.0031.
Higher Plasma Sclerostin and Lower Wnt Signaling Gene Expression in White Adipose Tissue of Prediabetic South Asian Men Compared with White Caucasian Men
- Affiliations
-
- 1Division of Endocrinology, Department of Medicine, Leiden University Medical Center, Leiden
- 2Einthoven Laboratory for Experimental Vascular Medicine, Leiden University Medical Center, Leiden
- 3Department of Human Biology and Movement Sciences, NUTRIM School for Nutrition and Translational Research in Metabolism, Maastricht University Medical Center, Maastricht
- 4Center for Bone Quality, Division of Endocrinology, Leiden University Medical Center, Leiden, The Netherlands
- KMID: 2502414
- DOI: http://doi.org/10.4093/dmj.2019.0031
Abstract
- Background
South Asians generally have an unfavourable metabolic phenotype compared with white Caucasians, including central obesity and insulin resistance. The Wnt protein family interacts with insulin signaling, and impaired Wnt signaling is associated with adiposity and type 2 diabetes mellitus. We aimed to investigate Wnt signaling in relation to insulin signaling in South Asians compared with white Caucasians.
Methods
Ten Dutch South Asian men with prediabetes and overweight or obesity and 10 matched Dutch white Caucasians were included. Blood samples were assayed for the Wnt inhibitor sclerostin. Subcutaneous white adipose tissue (WAT) and skeletal muscle biopsies were assayed for Wnt and insulin signaling gene expression with quantitative reverse transcription polymerase chain reaction (Clinicaltrials.gov NCT02291458).
Results
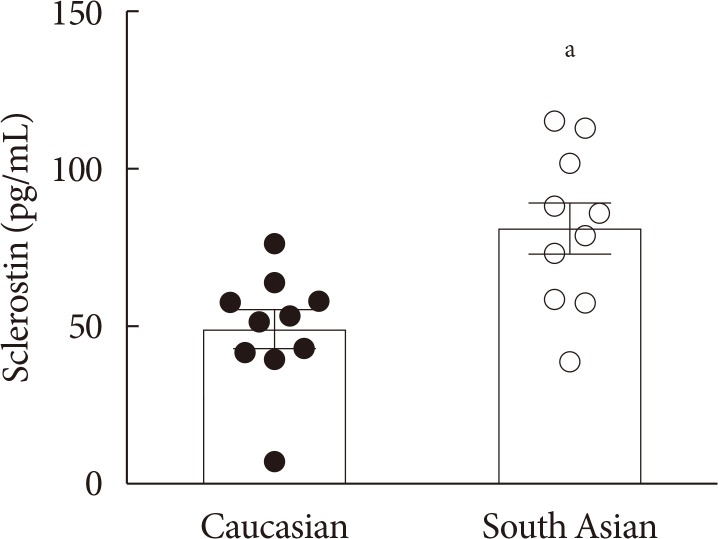
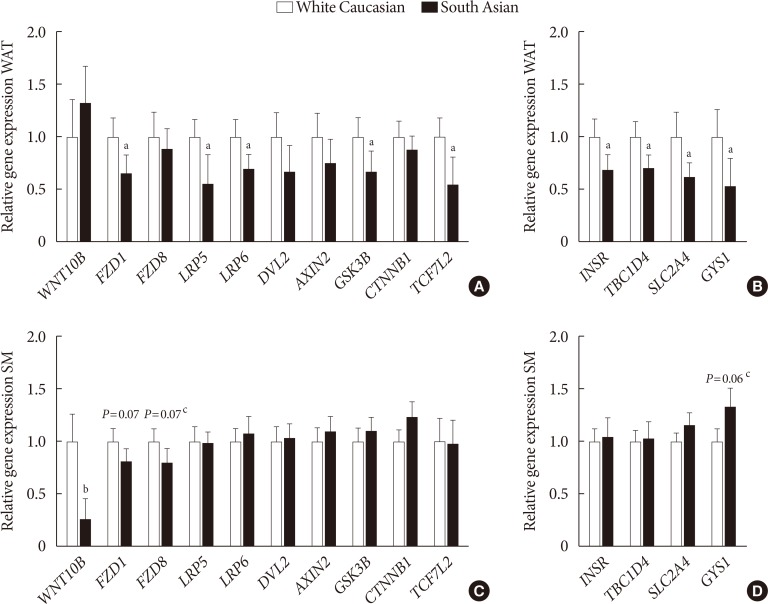
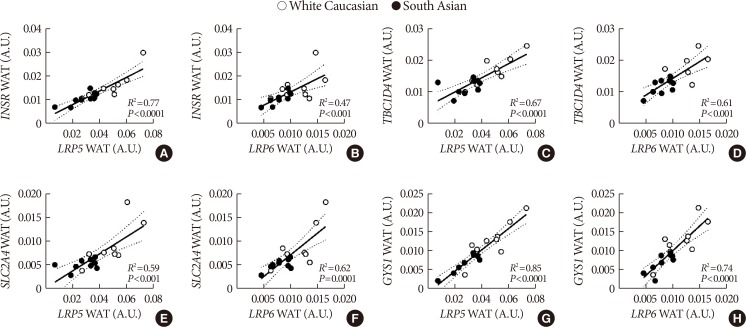
Plasma sclerostin was markedly higher in South Asians compared with white Caucasians (+65%, P<0.01). Additionally, expression of multiple Wnt signaling genes and key insulin signaling genes were lower in WAT in South Asians compared with white Caucasians. Moreover, in WAT in both ethnicities, Wnt signaling gene expression strongly positively correlated with insulin signaling gene expression. In skeletal muscle, WNT10B expression in South Asians was lower, but expression of other Wnt signaling and insulin signaling genes was comparable between ethnicities. Wnt and insulin signaling gene expression also positively correlated in skeletal muscle, albeit less pronounced.
Conclusion
South Asian men with overweight or obesity and prediabetes have higher plasma sclerostin and lower Wnt signaling gene expression in WAT compared with white Caucasians. We interpret that reduced Wnt signaling could contribute to impaired insulin signaling in South Asians.
Figure
Reference
-
1. Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res. 2016; 118:1723–1735. PMID: 27230638.
Article2. Middelkoop BJ, Kesarlal-Sadhoeram SM, Ramsaransing GN, Struben HW. Diabetes mellitus among South Asian inhabitants of the Hague: high prevalence and an age-specific socioeconomic gradient. Int J Epidemiol. 1999; 28:1119–1123. PMID: 10661656.
Article3. Meeks KA, Freitas-Da-Silva D, Adeyemo A, Beune EJ, Modesti PA, Stronks K, Zafarmand MH, Agyemang C. Disparities in type 2 diabetes prevalence among ethnic minority groups resident in Europe: a systematic review and meta-analysis. Intern Emerg Med. 2016; 11:327–340. PMID: 26370238.
Article4. Haldar S, Chia SC, Henry CJ. Body composition in Asians and Caucasians: comparative analyses and influences on cardiometabolic outcomes. Adv Food Nutr Res. 2015; 75:97–154. PMID: 26319906.5. Misra A, Ramchandran A, Jayawardena R, Shrivastava U, Snehalatha C. Diabetes in South Asians. Diabet Med. 2014; 31:1153–1162. PMID: 24975549.
Article6. McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991; 337:382–386. PMID: 1671422.
Article7. Kikuchi A, Yamamoto H, Kishida S. Multiplicity of the interactions of Wnt proteins and their receptors. Cell Signal. 2007; 19:659–671. PMID: 17188462.
Article8. Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000; 407:530–535. PMID: 11029007.
Article9. Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007; 315:1278–1282. PMID: 17332414.
Article10. Saarinen A, Saukkonen T, Kivela T, Lahtinen U, Laine C, Somer M, Toiviainen-Salo S, Cole WG, Lehesjoki AE, Makitie O. Low density lipoprotein receptor-related protein 5 (LRP5) mutations and osteoporosis, impaired glucose metabolism and hypercholesterolaemia. Clin Endocrinol (Oxf). 2010; 72:481–488. PMID: 19673927.
Article11. Singh R, De Aguiar RB, Naik S, Mani S, Ostadsharif K, Wencker D, Sotoudeh M, Malekzadeh R, Sherwin RS, Mani A. LRP6 enhances glucose metabolism by promoting TCF7L2-dependent insulin receptor expression and IGF receptor stabilization in humans. Cell Metab. 2013; 17:197–209. PMID: 23395167.
Article12. Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007; 445:881–885. PMID: 17293876.
Article13. Daniele G, Winnier D, Mari A, Bruder J, Fourcaudot M, Pengou Z, Tripathy D, Jenkinson C, Folli F. Sclerostin and insulin resistance in prediabetes: evidence of a cross talk between bone and glucose metabolism. Diabetes Care. 2015; 38:1509–1517. PMID: 26084344.
Article14. Christodoulides C, Scarda A, Granzotto M, Milan G, Dalla Nora E, Keogh J, De Pergola G, Stirling H, Pannacciulli N, Sethi JK, Federspil G, Vidal-Puig A, Farooqi IS, O'Rahilly S, Vettor R. WNT10B mutations in human obesity. Diabetologia. 2006; 49:678–684. PMID: 16477437.
Article15. Guo YF, Xiong DH, Shen H, Zhao LJ, Xiao P, Guo Y, Wang W, Yang TL, Recker RR, Deng HW. Polymorphisms of the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with obesity phenotypes in a large family-based association study. J Med Genet. 2006; 43:798–803. PMID: 16723389.
Article16. Loh NY, Neville MJ, Marinou K, Hardcastle SA, Fielding BA, Duncan EL, McCarthy MI, Tobias JH, Gregson CL, Karpe F, Christodoulides C. LRP5 regulates human body fat distribution by modulating adipose progenitor biology in a dose- and depot-specific fashion. Cell Metab. 2015; 21:262–273. PMID: 25651180.
Article17. Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A. Adipogenesis and WNT signalling. Trends Endocrinol Metab. 2009; 20:16–24. PMID: 19008118.
Article18. United Nations Statistics Division: Methodology: standard country or area codes for statistical use (M49). cited 2019 Sep 9. Available from: https://unstats.un.org/unsd/methodology/m49/.19. American Diabetes Association. Standards of medical care in diabetes: 2014. Diabetes Care. 2014; 37 Suppl 1:S14–S80. PMID: 24357209.20. van Lierop AH, Hamdy NA, Hamersma H, van Bezooijen RL, Power J, Loveridge N, Papapoulos SE. Patients with sclerosteosis and disease carriers: human models of the effect of sclerostin on bone turnover. J Bone Miner Res. 2011; 26:2804–2811. PMID: 21786318.
Article21. Weivoda MM, Youssef SJ, Oursler MJ. Sclerostin expression and functions beyond the osteocyte. Bone. 2017; 96:45–50. PMID: 27888056.
Article22. Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005; 280:19883–19887. PMID: 15778503.
Article23. Appelman-Dijkstra NM, Papapoulos SE. Clinical advantages and disadvantages of anabolic bone therapies targeting the WNT pathway. Nat Rev Endocrinol. 2018; 14:605–623. PMID: 30181608.
Article24. Chang YC, Hsu BG, Liou HH, Lee CJ, Wang JH. Serum levels of sclerostin as a potential biomarker in central arterial stiffness among hypertensive patients. BMC Cardiovasc Disord. 2018; 18:214. PMID: 30482161.
Article25. Garcia-Martin A, Rozas-Moreno P, Reyes-Garcia R, Morales-Santana S, Garcia-Fontana B, Garcia-Salcedo JA, Munoz-Torres M. Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012; 97:234–241. PMID: 22031520.26. Gaudio A, Privitera F, Battaglia K, Torrisi V, Sidoti MH, Pulvirenti I, Canzonieri E, Tringali G, Fiore CE. Sclerostin levels associated with inhibition of the Wnt/β-catenin signaling and reduced bone turnover in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012; 97:3744–3750. PMID: 22855334.
Article27. Yu OH, Richards B, Berger C, Josse RG, Leslie WD, Goltzman D, Kaiser SM, Kovacs CS, Davison KS. The association between sclerostin and incident type 2 diabetes risk: a cohort study. Clin Endocrinol (Oxf). 2017; 86:520–525. PMID: 28090669.
Article28. Faienza MF, Ventura A, Delvecchio M, Fusillo A, Piacente L, Aceto G, Colaianni G, Colucci S, Cavallo L, Grano M, Brunetti G. High sclerostin and dickkopf-1 (DKK-1) serum levels in children and adolescents with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2017; 102:1174–1181. PMID: 28388723.
Article29. Gennari L, Merlotti D, Valenti R, Ceccarelli E, Ruvio M, Pietrini MG, Capodarca C, Franci MB, Campagna MS, Calabro A, Cataldo D, Stolakis K, Dotta F, Nuti R. Circulating sclerostin levels and bone turnover in type 1 and type 2 diabetes. J Clin Endocrinol Metab. 2012; 97:1737–1744. PMID: 22399511.
Article30. Wedrychowicz A, Sztefko K, Starzyk JB. Sclerostin and its significance for children and adolescents with type 1 diabetes mellitus (T1D). Bone. 2019; 120:387–392. PMID: 30120991.
Article31. Kim SP, Frey JL, Li Z, Kushwaha P, Zoch ML, Tomlinson RE, Da H, Aja S, Noh HL, Kim JK, Hussain MA, Thorek DLJ, Wolfgang MJ, Riddle RC. Sclerostin influences body composition by regulating catabolic and anabolic metabolism in adipocytes. Proc Natl Acad Sci U S A. 2017; 114:E11238–E11247. PMID: 29229807.
Article32. Ukita M, Yamaguchi T, Ohata N, Tamura M. Sclerostin enhances adipocyte differentiation in 3T3-L1 cells. J Cell Biochem. 2016; 117:1419–1428. PMID: 26553151.
Article33. Karczewska-Kupczewska M, Stefanowicz M, Matulewicz N, Nikolajuk A, Straczkowski M. Wnt signaling genes in adipose tissue and skeletal muscle of humans with different degrees of insulin sensitivity. J Clin Endocrinol Metab. 2016; 101:3079–3087. PMID: 27218273.34. Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011; 12:722–734. PMID: 21952300.
Article35. van Dam AD, Hanssen MJW, van Eenige R, Quinten E, Sips HC, Hulsman CJM, Jazet IM, van Marken Lichtenbelt WD, Ottenhoff THM, Haks MC, Rensen PCN, Boon MR. South Asian men have lower expression of IFN signalling genes in white adipose tissue and skeletal muscle compared with white men. Diabetologia. 2017; 60:2525–2528. PMID: 28887664.
Article36. Mente A, Razak F, Blankenberg S, Vuksan V, Davis AD, Miller R, Teo K, Gerstein H, Sharma AM, Yusuf S, Anand SS. Study of the Health Assessment And Risk Evaluation in Aboriginal Peoples Investigators. Ethnic variation in adiponectin and leptin levels and their association with adiposity and insulin resistance. Diabetes Care. 2010; 33:1629–1634. PMID: 20413520.
Article37. Munoz A, Abate N, Chandalia M. Adipose tissue collagen and inflammation in nonobese Asian Indian men. J Clin Endocrinol Metab. 2013; 98:E1360–E1363. PMID: 23780376.
Article38. Boon MR, Hanssen MJW, Brans B, Hulsman CJM, Hoeks J, Nahon KJ, Bakker C, van Klinken JB, Havekes B, Schaart G, Jazet IM, Rensen PCN, van Marken Lichtenbelt WD. Effect of L-arginine on energy metabolism, skeletal muscle and brown adipose tissue in South Asian and Europid prediabetic men: a randomised double-blinded crossover study. Diabetologia. 2019; 62:112–122. PMID: 30377712.
Article39. Ghouri N, Purves D, McConnachie A, Wilson J, Gill JM, Sattar N. Lower cardiorespiratory fitness contributes to increased insulin resistance and fasting glycaemia in middle-aged South Asian compared with European men living in the UK. Diabetologia. 2013; 56:2238–2249. PMID: 23811809.
Article40. Palsgaard J, Emanuelli B, Winnay JN, Sumara G, Karsenty G, Kahn CR. Cross-talk between insulin and Wnt signaling in preadipocytes. Role of Wnt co-receptor LDL receptor-related protein-5 (LRP5). J Biol Chem. 2016; 291:16878. PMID: 27496960.
Article41. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004; 363:157–163. PMID: 14726171.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Emerging Importance of Mitochondria in White Adipocytes: Neither Last nor Least
- High-fat diet alters the thermogenic gene expression to β-agonists or 18-carbon fatty acids in adipocytes derived from the white and brown adipose tissue of mice
- HOXC10 suppresses browning of white adipose tissues
- The Relation between Inhibition of Adipocyte Differentiation by Activation of Wnt Signaling and Impaired Glucose Metabolism in Human
- The Prevalence and Types of Androgenetic Alopecia in Korean