Acute Crit Care.
2020 May;35(2):93-101. 10.4266/acc.2019.00773.
Measurement of mean systemic filling pressure after severe hemorrhagic shock in swine anesthetized with propofol-based total intravenous anesthesia: implications for vasopressor-free resuscitation
- Affiliations
-
- 1Department of Anesthesiology, Faculty of Medicine, School of Health Sciences, University of Thessaly, Larissa
- 2Hellenic Society of Cardiopulmonary Resuscitation, Athens
- 33rd Department of Internal Medicine, Nikaia General Hospital, Nikaia
- 4ELPEN, Experimental-Research Centre, Athens, Greece
- 5European University Cyprus, School of Medicine, Nicosia, Cyprus
- KMID: 2502339
- DOI: http://doi.org/10.4266/acc.2019.00773
Abstract
- Background
Mean systemic filling pressure (Pmsf) is a quantitative measurement of a patient’s volume status and represents the tone of the venous reservoir. The aim of this study was to estimate Pmsf after severe hemorrhagic shock and cardiac arrest in swine anesthetized with propofol-based total intravenous anesthesia, as well as to evaluate Pmsf’s association with vasopressor-free resuscitation.
Methods
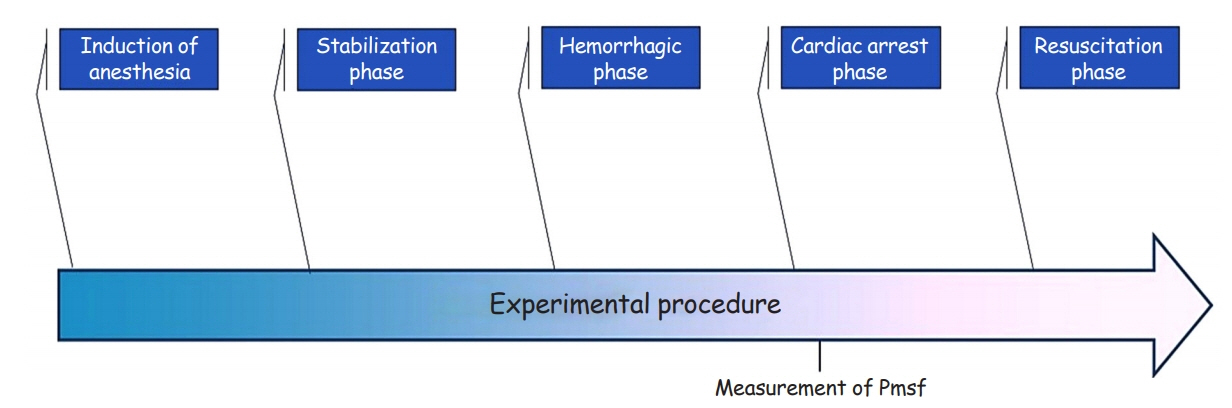
Ten healthy Landrace/Large-White piglets aged 10–12 weeks with average weight 20±1 kg were used in this study. The protocol was divided into four distinct phases: stabilization, hemorrhagic, cardiac arrest, and resuscitation phases. We measured Pmsf at 5–7.5 seconds after the onset of cardiac arrest and then every 10 seconds until 1 minute postcardiac arrest. During resuscitation, lactated Ringers was infused at a rate that aimed for a mean right atrial pressure of ≤4 mm Hg. No vasopressors were used.
Results
The mean volume of blood removed was 860±20 ml (blood loss, ~61%) and the bleeding time was 43.2±2 minutes while all animals developed pulseless electrical activity. Mean Pmsf was 4.09±1.22 mm Hg, and no significant differences in Pmsf were found until 1 minute postcardiac arrest (4.20±0.22 mm Hg at 5–7.5 seconds and 3.72±0.23 mm Hg at 55– 57.5 seconds; P=0.102). All animals achieved return of spontaneous circulation (ROSC), with mean time to ROSC being 6.1±1.7 minutes and mean administered volume being 394±20 ml.
Conclusions
For the first time, Pmsf was estimated after severe hemorrhagic shock. In this study, Pmsf remained stable during the first minute post-arrest. All animals achieved ROSC with goal-directed fluid resuscitation and no vasopressors.
Figure
Reference
-
1. Gidwani H, Gómez H. The crashing patient: hemodynamic collapse. Curr Opin Crit Care. 2017; 23:533–40.2. Guyton AC, Hall JE. Cardiac output, venous return, and their regulation. In : Schmitt W, Gruliow R, editors. Textbook of medical physiology. 10th ed. Philadelphia: W.B. Saunders; 2000;2000. p. 210–22.3. Aya HD, Cecconi M. Can (and should) the venous tone be monitored at the bedside? Curr Opin Crit Care. 2015; 21:240–4.
Article4. Schiller AM, Howard JT, Convertino VA. The physiology of blood loss and shock: new insights from a human laboratory model of hemorrhage. Exp Biol Med (Maywood). 2017; 242:874–83.
Article5. Shen T, Baker K. Venous return and clinical hemodynamics: how the body works during acute hemorrhage. Adv Physiol Educ. 2015; 39:267–71.
Article6. de Wit F, van Vliet AL, de Wilde RB, Jansen JR, Vuyk J, Aarts LP, et al. The effect of propofol on haemodynamics: cardiac output, venous return, mean systemic filling pressure, and vascular resistances. Br J Anaesth. 2016; 116:784–9.7. Al-Rifai Z, Mulvey D. Principles of total intravenous anaesthesia: basic pharmacokinetics and model descriptions. BJA Educ. 2016; 16:92–7.
Article8. Magder S. Volume and its relationship to cardiac output and venous return. Crit Care. 2016; 20:271.
Article9. Xanthos T, Lelovas P, Vlachos I, Tsirikos-Karapanos N, Kouskouni E, Perrea D, et al. Cardiopulmonary arrest and resuscitation in Landrace/Large White swine: a research model. Lab Anim. 2007; 41:353–62.
Article10. Xanthos T, Bassiakou E, Koudouna E, Rokas G, Goulas S, Dontas I, et al. Combination pharmacotherapy in the treatment of experimental cardiac arrest. Am J Emerg Med. 2009; 27:651–9.
Article11. Papapanagiotou P, Xanthos T, Gulati A, Chalkias A, Papalois A, Kontouli Z, et al. Centhaquin improves survival in a swine model of hemorrhagic shock. J Surg Res. 2016; 200:227–35.
Article12. Raffay V, Chalkias A, Lelovas P, Karlis G, Koutsovasilis A, Papalois A, et al. Addition of glucagon to adrenaline improves hemodynamics in a porcine model of prolonged ventricular fibrillation. Am J Emerg Med. 2014; 32:139–43.
Article13. Guyton AC, Polizo D, Armstrong GG. Mean circulatory filling pressure measured immediately after cessation of heart pumping. Am J Physiol. 1954; 179:261–7.
Article14. Chalkias A, Xanthos T. Pathophysiology and pathogenesis of post-resuscitation myocardial stunning. Heart Fail Rev. 2012; 17:117–28.
Article15. Ogilvie RI, Zborowska-Sluis D, Tenaschuk B. Measurement of mean circulatory filling pressure and vascular compliance in domestic pigs. Am J Physiol. 1990; 258(6 Pt 2):H1925–32.
Article16. Schipke JD, Heusch G, Sanii AP, Gams E, Winter J. Static filling pressure in patients during induced ventricular fibrillation. Am J Physiol Heart Circ Physiol. 2003; 285:H2510–5.
Article17. Fessler HE, Brower RG, Wise RA, Permutt S. Effects of positive end-expiratory pressure on the gradient for venous return. Am Rev Respir Dis. 1991; 143:19–24.
Article18. Jellinek H, Krenn H, Oczenski W, Veit F, Schwarz S, Fitzgerald RD. Influence of positive airway pressure on the pressure gradient for venous return in humans. J Appl Physiol (1985). 2000; 88:926–32.19. Aya HD, Carsetti A, Bazurro S, Bastoni D, Malbrain ML, Cecconi M. From cardiac output to blood flow auto-regulation in shock. Anaesthesiol Intensive Ther. 2015; 47 Spec No:s56-62.
Article20. Maas JJ. Mean systemic filling pressure: its measurement and meaning. Netherlands J Crit Care. 2015; 19:6–11.21. Samar RE, Coleman TG. Measurement of mean circulatory filling pressure and vascular capacitance in the rat. Am J Physiol. 1978; 234:H94–100.
Article22. Aya HD, Cecconi M. Determinants of Venous Return. In : Pinsky MR, Teboul JL, Vincent JL, editors. Hemodynamic monitoring: lessons from the ICU. Switzerland: Springer Nature;2019. p. 27–37.23. Rothe CF. Mean circulatory filling pressure: its meaning and measurement. J Appl Physiol (1985). 1993; 74:499–509.
Article24. Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016; 20:100.
Article25. Gupta B, Garg N, Ramachandran R. Vasopressors: do they have any role in hemorrhagic shock? J Anaesthesiol Clin Pharmacol. 2017; 33:3–8.
Article26. Yamamoto R, Suzuki M, Hayashida K, Yoshizawa J, Sakurai A, Kitamura N, et al. Epinephrine during resuscitation of traumatic cardiac arrest and increased mortality: a post hoc analysis of prospective observational study. Scand J Trauma Resusc Emerg Med. 2019; 27:74.
Article27. Kinsky M, Ribeiro N, Cannesson M, Deyo D, Kramer G, Salter M, et al. Peripheral venous pressure as an indicator of preload responsiveness during volume resuscitation from hemorrhage. Anesth Analg. 2016; 123:114–22.
Article28. Hall JE. Cardiac output, venous return, and their regulation. In : Hall JE, editor. Guyton and Hall textbook of medical physiology. 13th ed. Philadelphia: Elsevier;2016. p. 245–58.29. Hoka S, Yamaura K, Takenaka T, Takahashi S. Propofol-induced increase in vascular capacitance is due to inhibition of sympathetic vasoconstrictive activity. Anesthesiology. 1998; 89:1495–500.
Article30. Ebert TJ. Sympathetic and hemodynamic effects of moderate and deep sedation with propofol in humans. Anesthesiology. 2005; 103:20–4.
Article31. Robinson BJ, Ebert TJ, O’Brien TJ, Colinco MD, Muzi M. Mechanisms whereby propofol mediates peripheral vasodilation in humans: sympathoinhibition or direct vascular relaxation? Anesthesiology. 1997; 86:64–72.32. Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014; 40:1795–815.
Article33. Papalexopoulou K, Chalkias A, Pliatsika P, Papalois A, Papapanagiotou P, Papadopoulos G, et al. Centhaquin effects in a swine model of ventricular fibrillation: centhaquin and cardiac arrest. Heart Lung Circ. 2017; 26:856–63.34. Kontouli Z, Staikou C, Iacovidou N, Mamais I, Kouskouni E, Papalois A, et al. Resuscitation with centhaquin and 6% hydroxyethyl starch 130/0.4 improves survival in a swine model of hemorrhagic shock: a randomized experimental study. Eur J Trauma Emerg Surg. 2019; 45:1077–85.
Article35. Gulati A, Jain D, Agrawal N, Rahate P, Das S, Chowdhuri R, et al. Clinical phase II results of pmz-2010 (centhaquin) as a resuscitative agent for hypovolemic shock. Crit Care Med. 2019; 47:12.36. da Luz VF, Otsuki DA, Gonzalez MM, Negri EM, Caldini EG, Damaceno-Rodrigues NR, et al. Myocardial protection induced by fentanyl in pigs exposed to high-dose adrenaline. Clin Exp Pharmacol Physiol. 2015; 42:1098–107.
Article37. Vasileiou I, Xanthos T, Koudouna E, Perrea D, Klonaris C, Katsargyris A, et al. Propofol: a review of its non-anaesthetic effects. Eur J Pharmacol. 2009; 605:1–8.
Article38. Henderson WR, Griesdale DE, Walley KR, Sheel AW. Clinical review. Guyton: the role of mean circulatory filling pressure and right atrial pressure in controlling cardiac output. Crit Care. 2010; 14:243.39. Cockshott ID, Douglas EJ, Plummer GF, Simons PJ. The pharmacokinetics of propofol in laboratory animals. Xenobiotica. 1992; 22:369–75.
Article40. De Paepe P, Belpaire FM, Rosseel MT, Van Hoey G, Boon PA, Buylaert WA. Influence of hypovolemia on the pharmacokinetics and the electroencephalographic effect of propofol in the rat. Anesthesiology. 2000; 93:1482–90.
Article41. Johnson KB, Egan TD, Kern SE, White JL, McJames SW, Syroid N, et al. The influence of hemorrhagic shock on propofol: a pharmacokinetic and pharmacodynamic analysis. Anesthesiology. 2003; 99:409–20.42. Johnson KB, Egan TD, Kern SE, McJames SW, Cluff ML, Pace NL. Influence of hemorrhagic shock followed by crystalloid resuscitation on propofol: a pharmacokinetic and pharmacodynamic analysis. Anesthesiology. 2004; 101:647–59.43. Chalkias A, Arnaoutoglou E, Xanthos T. Personalized physiology-guided resuscitation in highly monitored patients with cardiac arrest: the PERSEUS resuscitation protocol. Heart Fail Rev. 2019; 24:473–80.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Phenylephrine on Cardiac Performance and Myocardial Oxygen Balance in Resuscitation from Hemorrhagic Shock
- Target controlled infusion for total intravenous anesthesia in children
- Concerns of the anesthesiologist: anesthetic induction in severe sepsis or septic shock patients
- Effect of Remifentanil Concentration on Propofol Demand during Total Intravenous Anesthesia
- Low Dose Ketamine or Fentanyl for Total Intravenous Anesthesia with Propofol for Stress Urinary Incontinence