J Pathol Transl Med.
2020 Jan;54(1):87-94. 10.4132/jptm.2019.10.14.
Analysis of the molecular subtypes of preoperative core needle biopsy and surgical specimens in invasive breast cancer
- Affiliations
-
- 1Department of Hospital Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Division of Medical Oncology, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Cancer Research Institute, The Catholic University of Korea, Seoul, Korea
- 4Department of Surgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 5Department of Radiology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2501596
- DOI: http://doi.org/10.4132/jptm.2019.10.14
Abstract
- Background
Accurate molecular classification of breast core needle biopsy (CNB) tissue is important for determining neoadjuvant systemic therapies for invasive breast cancer. The researchers aimed to evaluate the concordance rate (CR) of molecular subtypes between CNBs and surgical specimens.
Methods
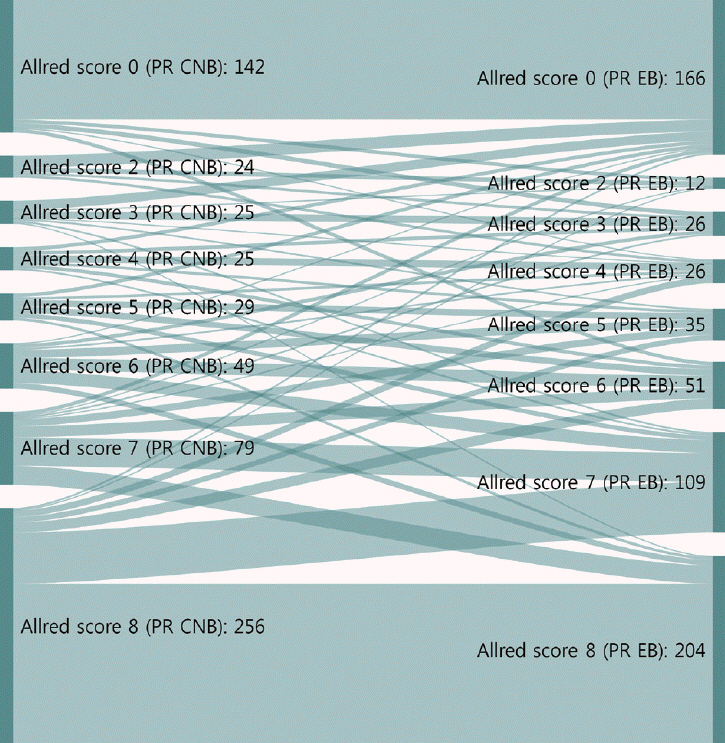
This study was conducted with invasive breast cancer patients who underwent surgery after CNB at Seoul St. Mary’s Hospital between December 2014 and December 2017. Estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki67 were analyzed using immunohistochemistry. ER and PR were evaluated by Allred score (0–8). HER2 was graded from 0 to +3, and all 2+ cases were reflex tested with silver in situ hybridization. The labeling index of Ki67 was counted by either manual scoring or digital image analysis. Molecular subtypes were classified using the above surrogate markers.
Results
In total, 629 patients were evaluated. The CRs of ER, PR, HER2, and Ki67 were 96.5% (kappa, 0.883; p<.001), 93.0% (kappa, 0.824; p<.001), 99.7% (kappa, 0.988; p<.001), and 78.7% (kappa, 0.577; p<.001), respectively. Digital image analysis of Ki67 in CNB showed better concordance with Ki67 in surgical specimens (CR, 82.3%; kappa, 0.639 for digital image analysis vs. CR, 76.2%; kappa, 0.534 for manual counting). The CRs of luminal A, luminal B, HER2, and triple negative types were 89.0%, 70.0%, 82.9%, and 77.2%, respectively.
Conclusions
CNB was reasonably accurate for determining ER, PR, HER2, Ki67, and molecular subtypes. Using digital image analysis for Ki67 in CNB produced more accurate molecular classifications.
Keyword
Figure
Reference
-
1. Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009; 101:736–50.
Article2. Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013; 24:2206–23.3. Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–47.4. Neubauer H, Gall C, Vogel U, et al. Changes in tumour biological markers during primary systemic chemotherapy (PST). Anticancer Res. 2008; 28:1797–804.5. Untch M, Konecny GE, Paepke S, von Minckwitz G. Current and future role of neoadjuvant therapy for breast cancer. Breast. 2014; 23:526–37.
Article6. Teshome M, Hunt KK. Neoadjuvant therapy in the treatment of breast cancer. Surg Oncol Clin N Am. 2014; 23:505–23.
Article7. Loibl S, Denkert C, von Minckwitz G. Neoadjuvant treatment of breast cancer: clinical and research perspective. Breast. 2015; 24 Suppl 2:S73–7.8. Ge WK, Yang B, Zuo WS, et al. Evaluation of hormone receptor, human epidermal growth factor receptor-2 and Ki-67 with core needle biopsy and neoadjuvant chemotherapy effects in breast cancer patients. Thorac Cancer. 2015; 6:64–9.
Article9. Piper GL, Patel NA, Patel JA, Malay MB, Julian TB. Neoadjuvant chemotherapy for locally advanced breast cancer results in alterations in preoperative tumor marker status. Am Surg. 2004; 70:1103–6.10. Meattini I, Bicchierai G, Saieva C, et al. Impact of molecular subtypes classification concordance between preoperative core needle biopsy and surgical specimen on early breast cancer management: single-institution experience and review of published literature. Eur J Surg Oncol. 2017; 43:642–8.
Article11. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998; 11:155–68.12. Wolff AC, Hammond ME, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018; 36:2105–22.13. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013; 31:3997–4013.14. Cho U, Kim HE, Oh WJ, Yeo MK, Song BJ, Lee A. The long-term prognostic performance of Ki-67 in primary operable breast cancer and evaluation of its optimal cutoff value. Appl Immunohistochem Mol Morphol. 2016; 24:159–66.
Article15. Dowsett M, Nielsen TO, A'Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011; 103:1656–64.16. Zidan A, Christie Brown JS, Peston D, Shousha S. Oestrogen and progesterone receptor assessment in core biopsy specimens of breast carcinoma. J Clin Pathol. 1997; 50:27–9.
Article17. Rakha EA, Ellis IO. An overview of assessment of prognostic and predictive factors in breast cancer needle core biopsy specimens. J Clin Pathol. 2007; 60:1300–6.
Article18. Park SY, Kim KS, Lee TG, et al. The accuracy of preoperative core biopsy in determining histologic grade, hormone receptors, and human epidermal growth factor receptor 2 status in invasive breast cancer. Am J Surg. 2009; 197:266–9.
Article19. Ricci MD, Calvano Filho CM, Oliveira Filho HR, Filassi JR, Pinotti JA, Baracat EC. Analysis of the concordance rates between core needle biopsy and surgical excision in patients with breast cancer. Rev Assoc Med Bras (1992). 2012; 58:532–6.
Article20. Ozdemir A, Voyvoda NK, Gultekin S, Tuncbilek I, Dursun A, Yamac D. Can core biopsy be used instead of surgical biopsy in the diagnosis and prognostic factor analysis of breast carcinoma? Clin Breast Cancer. 2007; 7:791–5.
Article21. Ough M, Velasco J, Hieken TJ. A comparative analysis of core needle biopsy and final excision for breast cancer: histology and marker expression. Am J Surg. 2011; 201:692–4.
Article22. Chen J, Wang Z, Lv Q, et al. Comparison of core needle biopsy and excision specimens for the accurate evaluation of breast cancer molecular markers: a report of 1003 cases. Pathol Oncol Res. 2017; 23:769–75.
Article23. You K, Park S, Ryu JM, et al. Comparison of core needle biopsy and surgical specimens in determining intrinsic biological subtypes of breast cancer with immunohistochemistry. J Breast Cancer. 2017; 20:297–303.
Article24. Ensani F, Omranipour R, Jahanzad I, Jafari A, Nafarzadeh S, Aminishakib P. The core needle and surgical biopsy concordance to detect estrogen, progesterone, and Her-2 receptors in breast cancer: a comparative study. Iran J Pathol. 2017; 12:202–8.
Article25. Liu M, Tang SX, Tsang JY, et al. Core needle biopsy as an alternative to whole section in IHC4 score assessment for breast cancer prognostication. J Clin Pathol. 2018; 71:1084–9.
Article26. Lorgis V, Algros MP, Villanueva C, et al. Discordance in early breast cancer for tumour grade, estrogen receptor, progesteron receptors and human epidermal receptor-2 status between core needle biopsy and surgical excisional primary tumour. Breast. 2011; 20:284–7.
Article27. Chen X, Sun L, Mao Y, et al. Preoperative core needle biopsy is accurate in determining molecular subtypes in invasive breast cancer. BMC Cancer. 2013; 13:390.
Article28. Usami S, Moriya T, Amari M, et al. Reliability of prognostic factors in breast carcinoma determined by core needle biopsy. Jpn J Clin Oncol. 2007; 37:250–5.
Article29. Robertson S, Ronnlund C, de Boniface J, Hartman J. Re-testing of predictive biomarkers on surgical breast cancer specimens is clinically relevant. Breast Cancer Res Treat. 2019; 174:795–805.
Article30. Mazari FA, Sharma N, Dodwell D, Horgan K. Human epidermal growth factor 2-positive breast cancer with mammographic microcalcification: relationship to pathologic complete response after neoadjuvant chemotherapy. Radiology. 2018; 288:366–74.
Article31. Nie Z, Wang J, Ji XC. Microcalcification-associated breast cancer: HER2-enriched molecular subtype is associated with mammographic features. Br J Radiol. 2018; Jun. 21. [Epub]. https://doi.org/10.1259/bjr.20170942.
Article32. Fishman JE, Milikowski C, Ramsinghani R, Velasquez MV, Aviram G. US-guided core-needle biopsy of the breast: how many specimens are necessary? Radiology. 2003; 226:779–82.
Article33. McIlhenny C, Doughty JC, George WD, Mallon EA. Optimum number of core biopsies for accurate assessment of histological grade in breast cancer. Br J Surg. 2002; 89:84–5.
Article34. Greer LT, Rosman M, Mylander WC, et al. Does breast tumor heterogeneity necessitate further immunohistochemical staining on surgical specimens? J Am Coll Surg. 2013; 216:239–51.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinicopathologic Features of the Papillary Breast Lesions Diagnosed on Ultrasonography-guided Core Needle Biopsy
- Pseudoaneurysm of the Breast after Core Needle Biopsy: A Case Report
- Track Seeding in a Breast Cancer Patient after a 14-Gauge Core Needle Biopsy: A Case Report
- Factors in the Breast Core Needle Biopsies of Atypical Ductal Hyperplasia that Can Predict Carcinoma in the Subsequent Surgical Excision Specimens
- Identifying breast cancer patients who require a double-check of preoperative core needle biopsy and postoperative surgical specimens to determine the molecular subtype of their tumor