Intest Res.
2020 Apr;18(2):219-228. 10.5217/ir.2019.00037.
Inhibition of plasminogen activator inhibitor-1 attenuates against intestinal fibrosis in mice
- Affiliations
-
- 1Center for Matrix Biology and Medicine, Tokai University School of Medicine, Kanagawa, Japan
- 2Department of Gastroenterology, Tokai University School of Medicine, Kanagawa, Japan
- 3Research Center for Regenerative Medicine, Tokai University School of Medicine, Kanagawa, Japan
- 4Department of Cell Transplantation and Regenerative Medicine, Tokai University School of Medicine, Kanagawa, Japan
- 5Department of Hematology and Oncology, Tokai University School of Medicine, Kanagawa, Japan
- 6Department of Immunology, Tokai University School of Medicine, Kanagawa, Japan
- 7Department of Regenerative Medicine, Tokai University School of Medicine, Kanagawa, Japan
- 8Division of Molecular Medicine and Therapy, Tohoku University Graduate School of Medicine, Sendai, Japan
- KMID: 2501387
- DOI: http://doi.org/10.5217/ir.2019.00037
Abstract
- Background/Aims
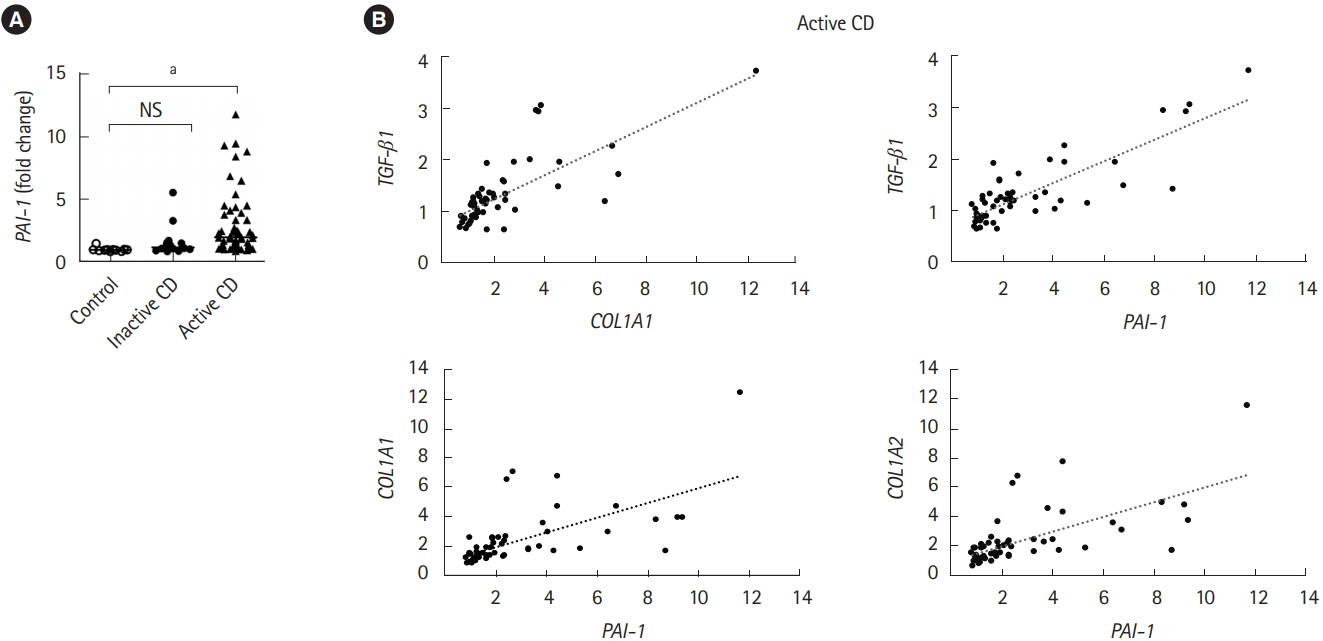
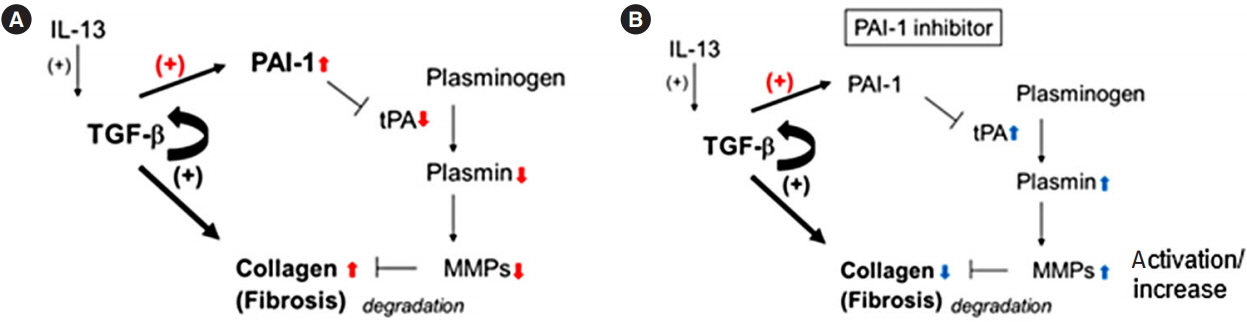
Intestinal fibrosis is a major complication of Crohn’s disease (CD). The profibrotic protein transforming growth factor-β (TGF-β) has been considered to be critical for the induction of the fibrotic program. TGF-β has the ability to induce not only the expression of extracellular matrix (ECM) including collagen, but also the production of plasminogen activator inhibitor-1 (PAI-1) that prevents enzymatic degradation of the ECM during the onset of fibrotic diseases. However, the significance of PAI-1 in the developing intestinal fibrosis has not been fully understood. In the present study, we examined the actual expression of PAI-1 in fibrotic legion of intestinal inflammation and its correlation with the abnormal ECM deposition.
Methods
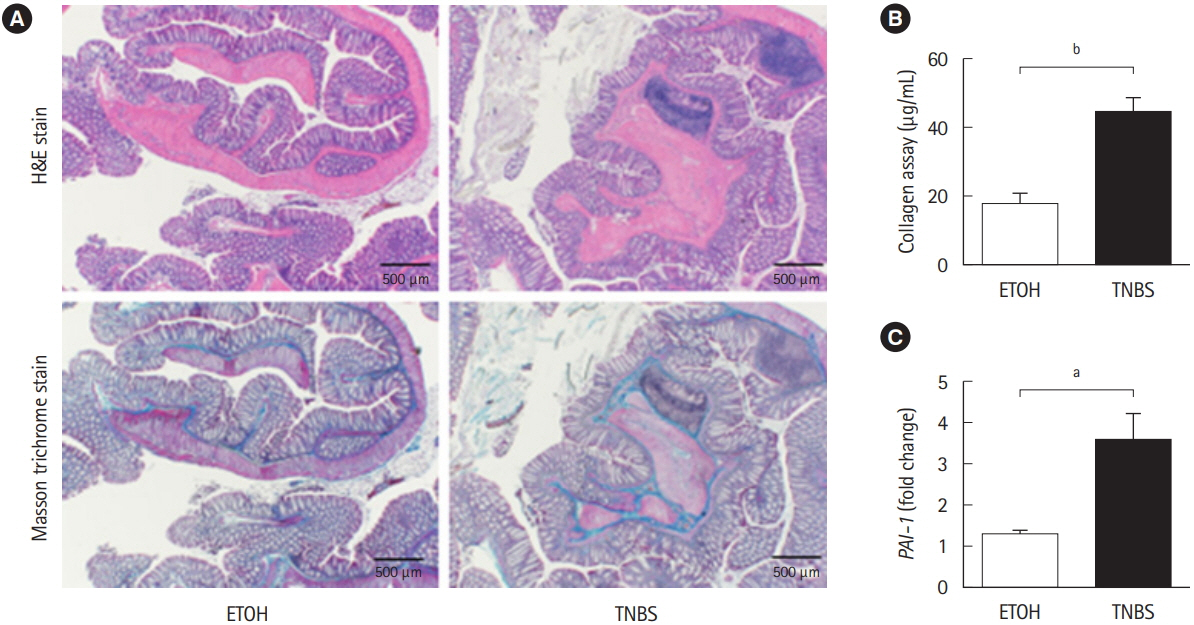
Chronic intestinal inflammation was induced in BALB/c mice using 8 repeated intrarectal injections of 2,4,6-trinitrobenzene sulfonic acid (TNBS). TM5275, a PAI-1 inhibitor, was orally administered as a carboxymethyl cellulose suspension each day for 2 weeks after the sixth TNBS injection.
Results
Using a publicly available dataset (accession number, GSE75214) and TNBS-treated mice, we observed increases in PAI-1 transcripts at active fibrotic lesions in both patients with CD and mice with chronic intestinal inflammation. Oral administration of TM5275 immediately after the onset of intestinal fibrosis upregulated MMP-9 (matrix metalloproteinase 9) and decreased collagen accumulation, resulting in attenuation of the fibrogenesis in TNBS-treated mice.
Conclusions
PAI-1-mediated fibrinolytic system facilitates collagen degradation suppression. Hence, PAI-1 inhibitor could be applied as an anti-fibrotic drug in CD treatment.
Keyword
Figure
Reference
-
1. Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011; 140:1785–1794.
Article2. Latella G, Sferra R, Speca S, Vetuschi A, Gaudio E. Can we prevent, reduce or reverse intestinal fibrosis in IBD? Eur Rev Med Pharmacol Sci. 2013; 17:1283–1304.3. Speca S, Giusti I, Rieder F, Latella G. Cellular and molecular mechanisms of intestinal fibrosis. World J Gastroenterol. 2012; 18:3635–3661.
Article4. Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology. 2017; 152:340–350.
Article5. Bettenworth D, Rieder F. Medical therapy of stricturing Crohn’s disease: what the gut can learn from other organs: a systematic review. Fibrogenesis Tissue Repair. 2014; 7:5.6. Verrecchia F, Mauviel A. Transforming growth factor-beta and fibrosis. World J Gastroenterol. 2007; 13:3056–3062.7. Imai J, Hozumi K, Sumiyoshi H, et al. Anti-fibrotic effects of a novel small compound on the regulation of cytokine production in a mouse model of colorectal fibrosis. Biochem Biophys Res Commun. 2015; 468:554–560.
Article8. Higashi K, Tomigahara Y, Shiraki H, et al. A novel small compound that promotes nuclear translocation of YB-1 ameliorates experimental hepatic fibrosis in mice. J Biol Chem. 2011; 286:4485–4492.
Article9. Samarakoon R, Higgins PJ. Integration of non-SMAD and SMAD signaling in TGF-beta1-induced plasminogen activator inhibitor type-1 gene expression in vascular smooth muscle cells. Thromb Haemost. 2008; 100:976–983.
Article10. Ghosh AK, Vaughan DE. PAI-1 in tissue fibrosis. J Cell Physiol. 2012; 227:493–507.
Article11. Kaiko GE, Chen F, Lai CW, et al. PAI-1 augments mucosal damage in colitis. Sci Transl Med. 2019; 11:e. –aat0852.
Article12. Ibrahim AA, Yahata T, Onizuka M, et al. Inhibition of plasminogen activator inhibitor type-1 activity enhances rapid and sustainable hematopoietic regeneration. Stem Cells. 2014; 32:946–958.
Article13. Latella G, Vetuschi A, Sferra R, et al. Smad3 loss confers resistance to the development of trinitrobenzene sulfonic acid-induced colorectal fibrosis. Eur J Clin Invest. 2009; 39:145–156.
Article14. Lawrance IC, Wu F, Leite AZ, et al. A murine model of chronic inflammation-induced intestinal fibrosis down-regulated by antisense NF-kappa B. Gastroenterology. 2003; 125:1750–1761.
Article15. Fichtner-Feigl S, Fuss IJ, Young CA, et al. Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol. 2007; 178:5859–5870.
Article16. Higashi K, Inagaki Y, Fujimori K, Nakao A, Kaneko H, Nakatsuka I. Interferon-gamma interferes with transforming growth factor-beta signaling through direct interaction of YB-1 with Smad3. J Biol Chem. 2003; 278:43470–43479.
Article17. Gasche C, Scholmerich J, Brynskov J, et al. A simple classification of Crohn’s disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000; 6:8–15.
Article18. Vancamelbeke M, Vanuytsel T, Farré R, et al. Genetic and transcriptomic bases of intestinal epithelial barrier dysfunction in inflammatory bowel disease. Inflamm Bowel Dis. 2017; 23:1718–1729.
Article19. Yahata T, Ibrahim AA, Muguruma Y, et al. TGF-beta-induced intracellular PAI-1 is responsible for retaining hematopoietic stem cells in the niche. Blood. 2017; 130:2283–2294.
Article20. Oh CK, Ariue B, Alban RF, Shaw B, Cho SH. PAI-1 promotes extracellular matrix deposition in the airways of a murine asthma model. Biochem Biophys Res Commun. 2002; 294:1155–1160.
Article21. Munakata S, Tashiro Y, Nishida C, et al. Inhibition of plasmin protects against colitis in mice by suppressing matrix metalloproteinase 9-mediated cytokine release from myeloid cells. Gastroenterology. 2015; 148:565–578.
Article22. Pincha N, Hajam EY, Badarinath K, et al. PAI1 mediates fibroblast-mast cell interactions in skin fibrosis. J Clin Invest. 2018; 128:1807–1819.
Article23. Rieder F, Bettenworth D, Imai J, Inagaki Y. Intestinal fibrosis and liver fibrosis: consequences of chronic inflammation or independent pathophysiology? Inflamm Intest Dis. 2016; 1:41–49.
Article24. Inagaki Y, Okazaki I. Emerging insights into transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007; 56:284–292.
Article25. Li C, Iness A, Yoon J, et al. Noncanonical STAT3 activation regulates excess TGF-beta1 and collagen I expression in muscle of stricturing Crohn’s disease. J Immunol. 2015; 194:3422–3431.
Article26. Diebold RJ, Eis MJ, Yin M, et al. Early-onset multifocal inflammation in the transforming growth factor beta 1-null mouse is lymphocyte mediated. Proc Natl Acad Sci U S A. 1995; 92:12215–12219.
Article27. Kulkarni AB, Ward JM, Yaswen L, et al. Transforming growth factor-beta 1 null mice: an animal model for inflammatory disorders. Am J Pathol. 1995; 146:264–275.28. Nomura M, Li E. Smad2 role in mesoderm formation, leftright patterning and craniofacial development. Nature. 1998; 393:786–790.
Article29. Yang X, Li C, Xu X, Deng C. The tumor suppressor SMAD4/ DPC4 is essential for epiblast proliferation and mesoderm induction in mice. Proc Natl Acad Sci U S A. 1998; 95:3667–3672.
Article30. de Bruyn M, Arijs I, Wollants WJ, et al. Neutrophil gelatinase B-associated lipocalin and matrix metalloproteinase-9 complex as a surrogate serum marker of mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2014; 20:1198–1207.
Article31. de Bruyn M, Vandooren J, Ugarte-Berzal E, Arijs I, Vermeire S, Opdenakker G. The molecular biology of matrix metalloproteinases and tissue inhibitors of metalloproteinases in inflammatory bowel diseases. Crit Rev Biochem Mol Biol. 2016; 51:295–358.
Article32. Castaneda FE, Walia B, Vijay-Kumar M, et al. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology. 2005; 129:1991–2008.
Article33. De Bruyn M, Breynaert C, Arijs I, et al. Inhibition of gelatinase B/MMP-9 does not attenuate colitis in murine models of inflammatory bowel disease. Nat Commun. 2017; 8:15384.
Article34. De Bruyn M, Ferrante M. Failure of MMP-9 antagonists in IBD: demonstrating the importance of molecular biology and well-controlled preclinical studies. J Crohns Colitis. 2018; 12:1011–1013.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Urokinase-type plasminogen activator receptor in IgA nephropathy
- Plasminogen Activator Inhibitor-1 in Asthma
- Effect of immune-mediated vascular injury on the coagulation- regulatory mechanism of the human endothelial cells; changes of tissue-type plasminogen activator, plasminogen activator inhibitor- 1 and von Willebrand factor
- Inhibition of Plasminogen Activator Inhibitor-1 Expression in Smoke-Exposed Alveolar Type II Epithelial Cells Attenuates Epithelial-Mesenchymal Transition
- Urokinase, urokinase receptor, and plasminogen activator inhibitor-1 expression on podocytes in immunoglobulin A glomerulonephritis