Intest Res.
2020 Jan;18(1):96-106. 10.5217/ir.2019.00092.
Clinical outcomes of submucosal colorectal cancer diagnosed after endoscopic resection: a focus on the need for surgery
- Affiliations
-
- 1Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Gyeongsang National University Changwon Hospital, Changwon, Korea
- KMID: 2501371
- DOI: http://doi.org/10.5217/ir.2019.00092
Abstract
- Background/Aims
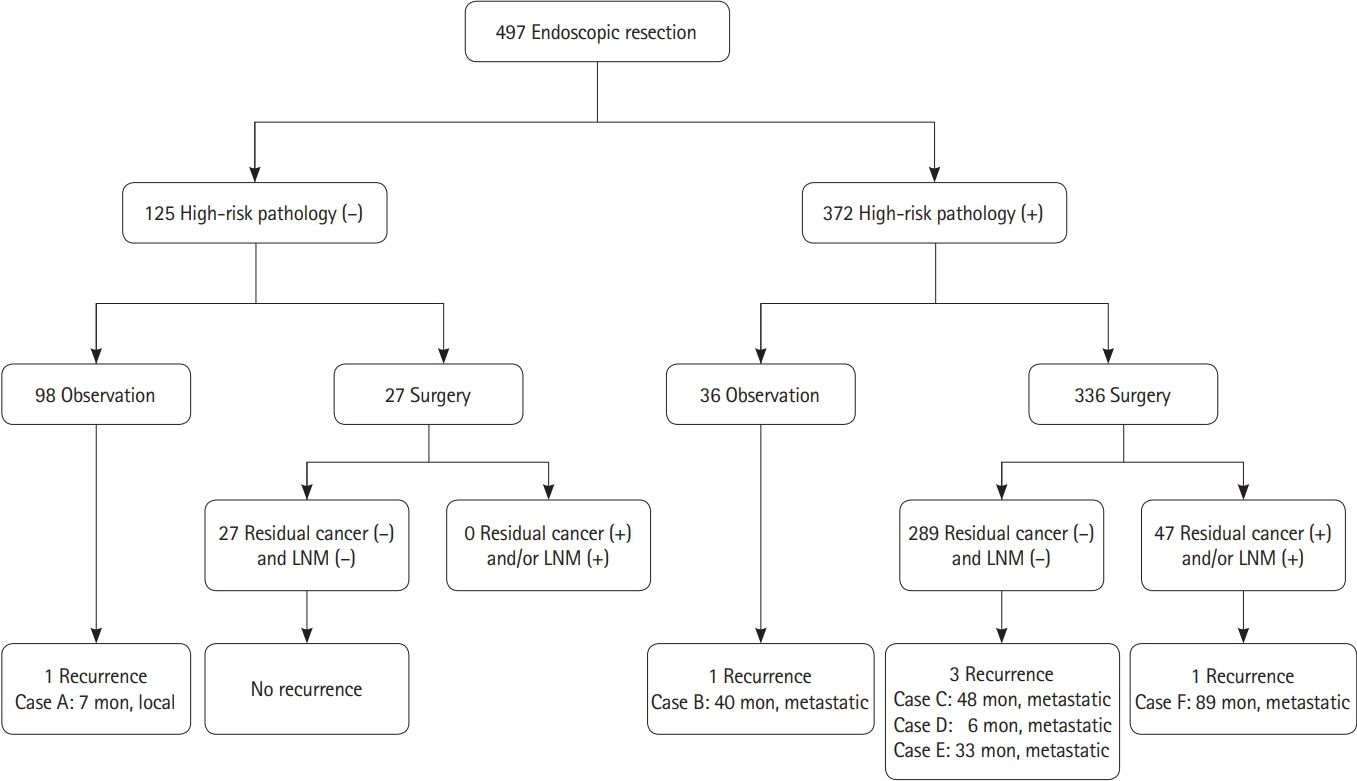
We aimed to investigate the proportion of and risk factors for residual cancer and/or lymph node metastasis after surgery was performed because of high-risk pathological features in endoscopic resection specimen of suspected superficial submucosal colorectal cancer (SSMC).
Methods
We reviewed medical records of 497 patients (58.8 ± 9.8 years, 331 males) undergoing endoscopic resection of suspected SSMC. High-risk pathological features included: deep submucosal cancer invasion ≥ 1,000 μm; positive lymphovascular and/or perineural invasion; poorly differentiated adenocarcinoma; and positive resection margin. We investigated the occurrence of additional surgery and residual cancer and/or lymph node involvement in the surgical specimen.
Results
En bloc resection was performed in 447 patients (89.9%). High-risk pathological features were detected in 372 patients (74.8%). Additional surgery was performed in 336 of 372 patients with high-risk pathological features. Of these, 47 surgical specimens (14.0%) showed residual cancer and/or lymph node metastasis. Piecemeal resection was more common in those with residual cancer and/or lymph node involvement than those without (9/47 [19.1%] vs. 24/289 [8.3%], P= 0.032). Positive resection margin was also significantly associated with positive residual cancer and/or lymph node involvement. As the number of high-risk pathological features increased, the risk of regional lymph node metastasis increased proportionally (P= 0.002).
Conclusions
High-risk pathological features were frequently detected after endoscopic resection of suspected SSMC while residual cancer and/or lymph node metastasis were not commonly present in the additional surgical specimen. Further optimized strategy for proper endoscopic management of suspected SSMC is necessary.
Figure
Cited by 1 articles
-
Endoscopic diagnosis and treatment of early colorectal cancer
Seung Wook Hong, Jeong-Sik Byeon
Intest Res. 2022;20(3):281-290. doi: 10.5217/ir.2021.00169.
Reference
-
1. Wong MC, Ding H, Wang J, Chan PS, Huang J. Prevalence and risk factors of colorectal cancer in Asia. Intest Res. 2019; 17:317–329.
Article2. Hong SN. Genetic and epigenetic alterations of colorectal cancer. Intest Res. 2018; 16:327–337.
Article3. Bevan R, Rutter MD. Colorectal cancer screening: who, how, and when? Clin Endosc. 2018; 51:37–49.4. Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2017; 85:2–21.
Article5. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017; 153:307–323.
Article6. Kim TJ, Kim ER, Hong SN, Kim YH, Chang DK. Current practices in endoscopic submucosal dissection for colorectal neoplasms: a survey of indications among Korean endoscopists. Intest Res. 2017; 15:228–235.
Article7. Kitajima K, Fujimori T, Fujii S, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004; 39:534–543.
Article8. Beaton C, Twine CP, Williams GL, Radcliffe AG. Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Colorectal Dis. 2013; 15:788–797.
Article9. Bosch SL, Teerenstra S, de Wilt JH, Cunningham C, Nagtegaal ID. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy. 2013; 45:827–834.
Article10. Mou S, Soetikno R, Shimoda T, Rouse R, Kaltenbach T. Pathologic predictive factors for lymph node metastasis in submucosal invasive (T1) colorectal cancer: a systematic review and meta-analysis. Surg Endosc. 2013; 27:2692–2703.
Article11. Ueno H, Mochizuki H, Hashiguchi Y, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004; 127:385–394.
Article12. Kim JB, Lee HS, Lee HJ, et al. Long-term outcomes of endoscopic versus surgical resection of superficial submucosal colorectal cancer. Dig Dis Sci. 2015; 60:2785–2792.
Article13. Watanabe T, Muro K, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018; 23:1–34.
Article14. Sano Y, Tanaka S, Kudo SE, et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc. 2016; 28:526–533.
Article15. Hayashi N, Tanaka S, Hewett DG, et al. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc. 2013; 78:625–632.
Article16. Puig I, López-Cerón M, Arnau A, et al. Accuracy of the narrow-band imaging international colorectal endoscopic classification system in identification of deep invasion in colorectal polyps. Gastroenterology. 2019; 156:75–87.17. Backes Y, Moss A, Reitsma JB, Siersema PD, Moons LM. Narrow band imaging, magnifying chromoendoscopy, and gross morphological features for the optical diagnosis of T1 colorectal cancer and deep submucosal invasion: a systematic review and meta-analysis. Am J Gastroenterol. 2017; 112:54–64.
Article18. Park J, Kim HG, Jeong SO, et al. Clinical outcomes of positive resection margin after endoscopic mucosal resection of early colon cancers. Intest Res. 2019; 17:516–526.
Article19. Huh JW, Kim HR, Kim YJ. Lymphovascular or perineural invasion may predict lymph node metastasis in patients with T1 and T2 colorectal cancer. J Gastrointest Surg. 2010; 14:1074–1080.
Article20. Betge J, Pollheimer MJ, Kornprat P, Rehak P, Vieth M, Langner C. Perineural invasion is a strong and independent predictor of lymph node involvement in colorectal cancer. Dis Colon Rectum. 2011; 54:e273.
Article21. Ikematsu H, Yoda Y, Matsuda T, et al. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology. 2013; 144:551–559.
Article22. Yoda Y, Ikematsu H, Matsuda T, et al. A large-scale multicenter study of long-term outcomes after endoscopic resection for submucosal invasive colorectal cancer. Endoscopy. 2013; 45:718–724.
Article23. Heo J, Jeon SW, Jung MK, Kim SK, Kim J, Kim S. Endoscopic resection as the first-line treatment for early colorectal cancer: comparison with surgery. Surg Endosc. 2014; 28:3435–3442.
Article24. Yoshii S, Nojima M, Nosho K, et al. Factors associated with risk for colorectal cancer recurrence after endoscopic resection of T1 tumors. Clin Gastroenterol Hepatol. 2014; 12:292–302.
Article25. Asayama N, Oka S, Tanaka S, et al. Long-term outcomes after treatment for T1 colorectal carcinoma. Int J Colorectal Dis. 2016; 31:571–578.
Article26. Nam MJ, Han KS, Kim BC, et al. Long-term outcomes of locally or radically resected T1 colorectal cancer. Colorectal Dis. 2016; 18:852–860.
Article27. Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2017; 49:270–297.
Article28. Moss A, Bourke MJ, Williams SJ, et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology. 2011; 140:1909–1918.
Article29. Seo M, Song EM, Kim GU, et al. Local recurrence and subsequent endoscopic treatment after endoscopic piecemeal mucosal resection with or without precutting in the colorectum. Intest Res. 2017; 15:502–510.
Article30. Woodward TA, Heckman MG, Cleveland P, De Melo S, Raimondo M, Wallace M. Predictors of complete endoscopic mucosal resection of flat and depressed gastrointestinal neoplasia of the colon. Am J Gastroenterol. 2012; 107:650–654.
Article31. Khashab M, Eid E, Rusche M, Rex DK. Incidence and predictors of “late” recurrences after endoscopic piecemeal resection of large sessile adenomas. Gastrointest Endosc. 2009; 70:344–349.
Article32. Kikuchi R, Takano M, Takagi K, et al. Management of early invasive colorectal cancer: risk of recurrence and clinical guidelines. Dis Colon Rectum. 1995; 38:1286–1295.33. Nascimbeni R, Burgart LJ, Nivatvongs S, Larson DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002; 45:200–206.
Article34. ASGE Standards of Practice Committee, Fisher DA, Shergill AK, et al. Role of endoscopy in the staging and management of colorectal cancer. Gastrointest Endosc. 2013; 78:8–12.
Article35. Labianca R, Nordlinger B, Beretta GD, et al. Early colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013; 24 Suppl 6:vi64–vi72.
Article36. Backes Y, Elias SG, Groen JN, et al. Histologic factors associated with need for surgery in patients with pedunculated T1 colorectal carcinomas. Gastroenterology. 2018; 154:1647–1659.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Challenges in Implementing Endoscopic Resection for T2 Colorectal Cancer
- Endoscopic diagnosis and treatment of early colorectal cancer
- Significance of rescue hybrid endoscopic submucosal dissection in difficult colorectal cases
- Endoscopic Treatment for Gastric Subepithelial Tumor
- Endoscopic Diagnosis and Treatment of Colorectal Cancers