Cancer Res Treat.
2020 Jan;52(1):320-333. 10.4143/crt.2019.124.
Prognostic Model for Survival and Recurrence in Patients with Early-Stage Cervical Cancer: A Korean Gynecologic Oncology Group Study (KGOG 1028)
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Cancer Healthcare Research Branch, Center for Uterine Cancer, and Center for Clinical Trials, Research Institute and Hospital and Cancer Control and Policy, Graduate School of Cancer Science and Policy, National Cancer Center, Goyang, Korea
- 3Department of Obstetrics and Gynecology, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences, Seoul, Korea
- 4Department of Obstetrics and Gynecology, Ewha Womans University Mokdong Hospital, Ewha Womans University School of Medicine, Seoul, Korea
- 5Medical Treatment Division, Gwangjin-gu Health Center, Seoul, Korea
- 6Department of Obstetrics and Gynecology, CHA Gangnam Medical Center, CHA University, Seoul, Korea
- 7Department of Obstetrics and Gynecology, Seoul National University Bundang Hospital, Seongnam, Korea
- 8Department of Obstetrics and Gynecology, Kyung Hee University Hospital at Gangdong, Kyung Hee University School of Medicine, Seoul, Korea
- 9Department of Obstetrics and Gynecology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea
- KMID: 2501224
- DOI: http://doi.org/10.4143/crt.2019.124
Abstract
- Purpose
We aimed to develop and validate individual prognostic models in a large cohort of cervical cancer patients that were primarily treated with radical hysterectomy.
Materials and Methods
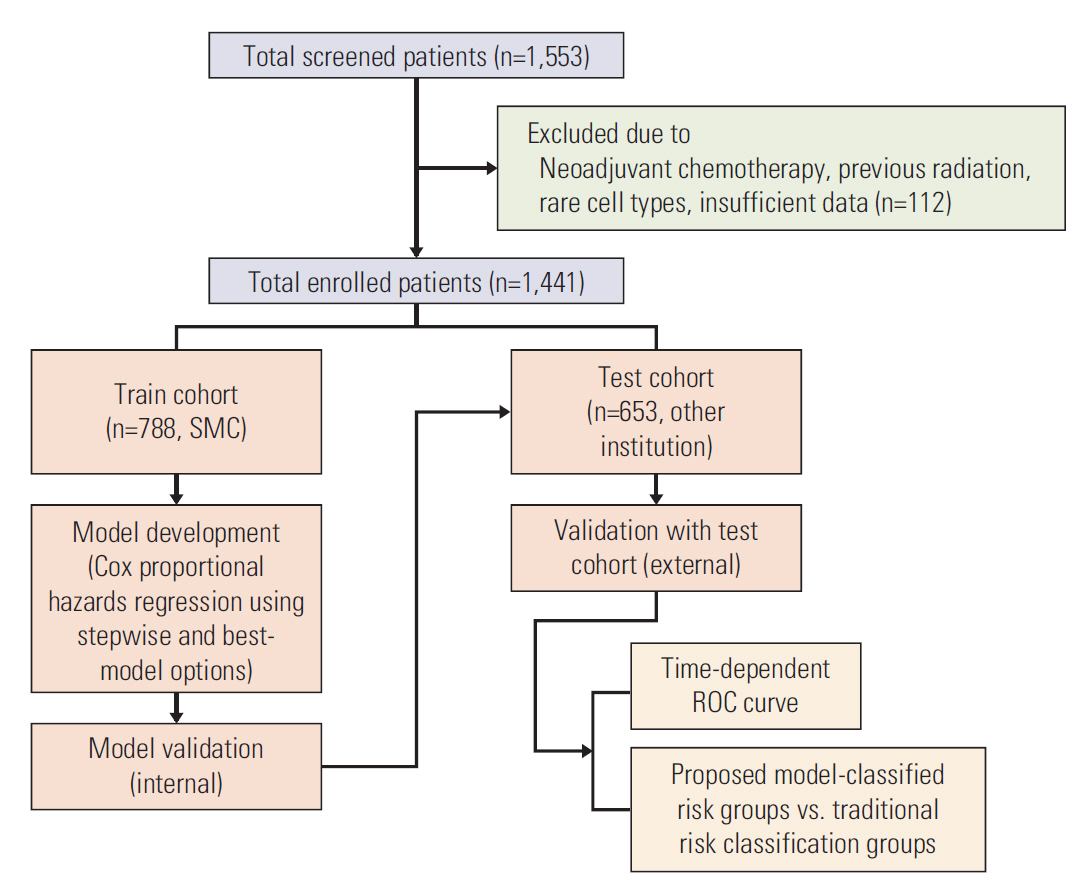
We analyzed 1,441 patients with early-stage cervical cancer treated between 2000 and 2008 from the Korean Gynecologic Oncology Group multi-institutional cohort: a train cohort (n=788) and a test cohort (n=653). Models predicting the risk for overall survival (OS), disease- free survival (DFS), lymphatic recurrence and hematogenous recurrence were developed using Cox analysis and stepwise backward selection and best-model options. The prognostic performance of each model was assessed in an independent patient cohort. Model-classified risk groups were compared to groups based on traditional risk factors.
Results
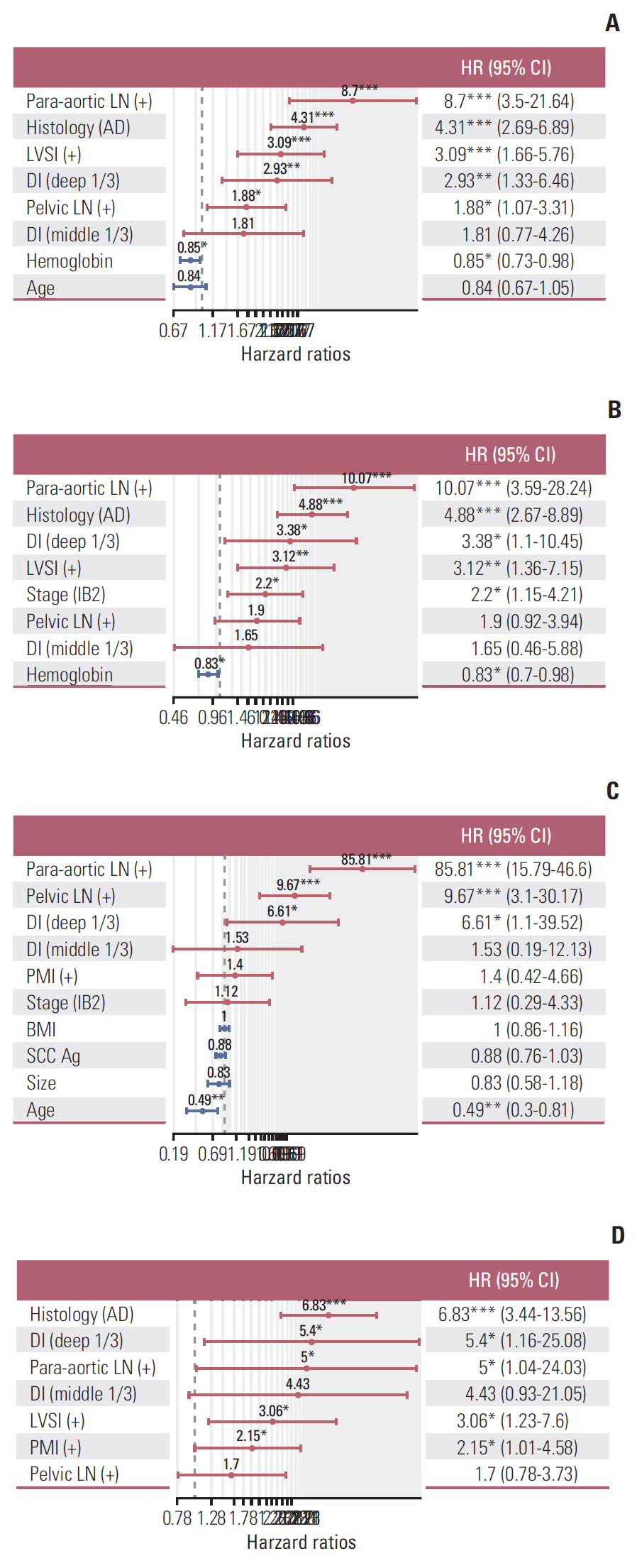
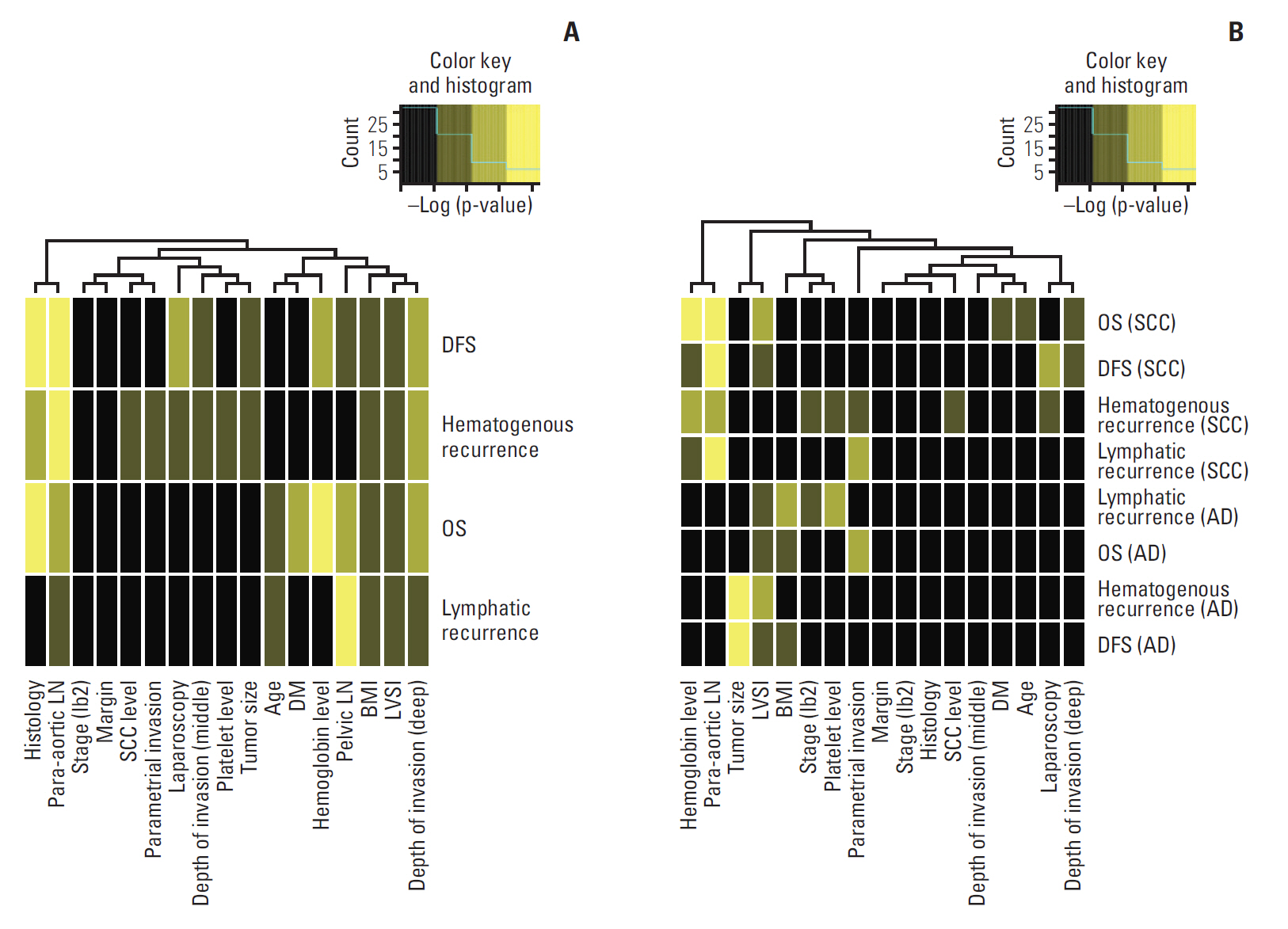
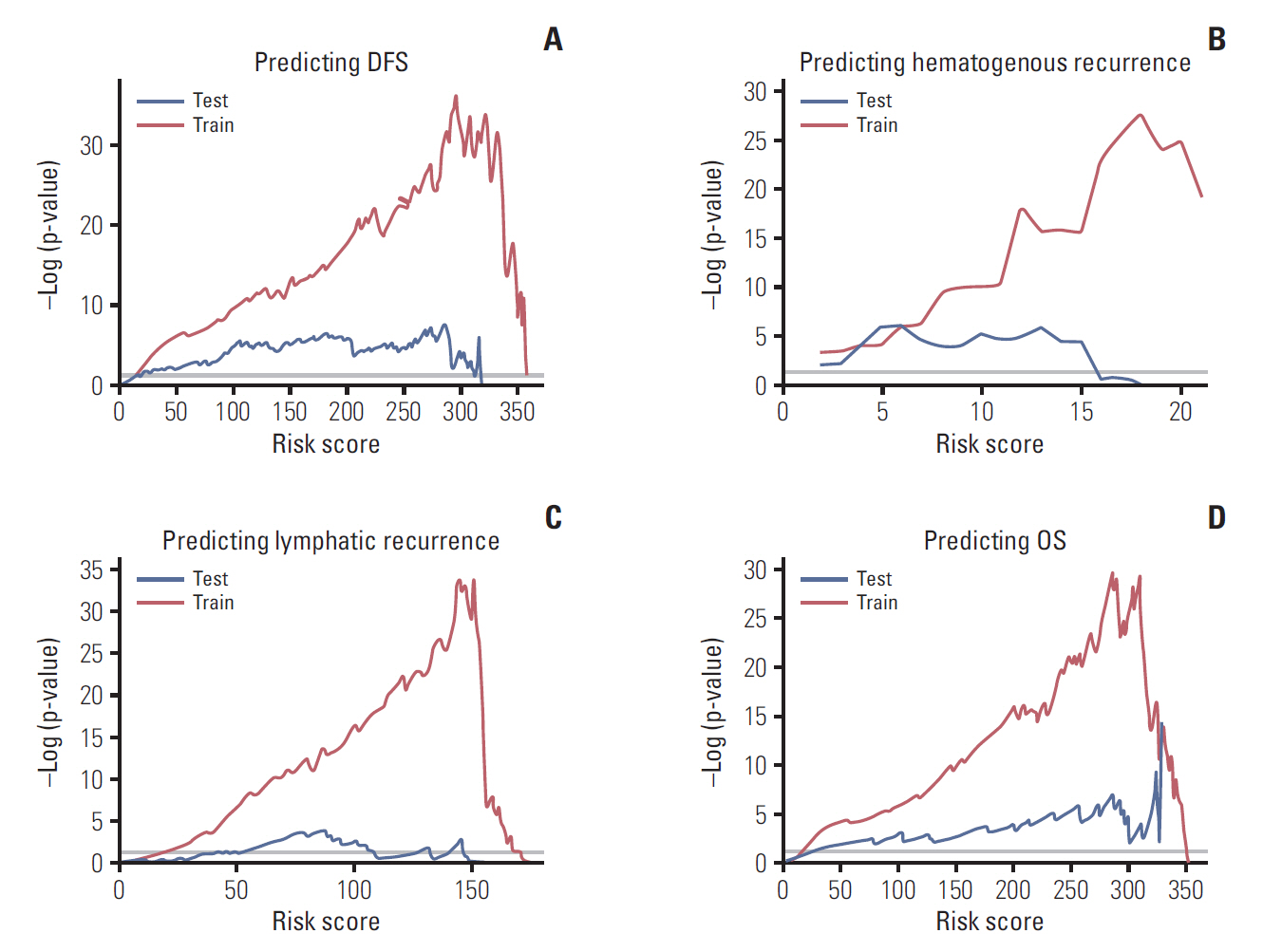
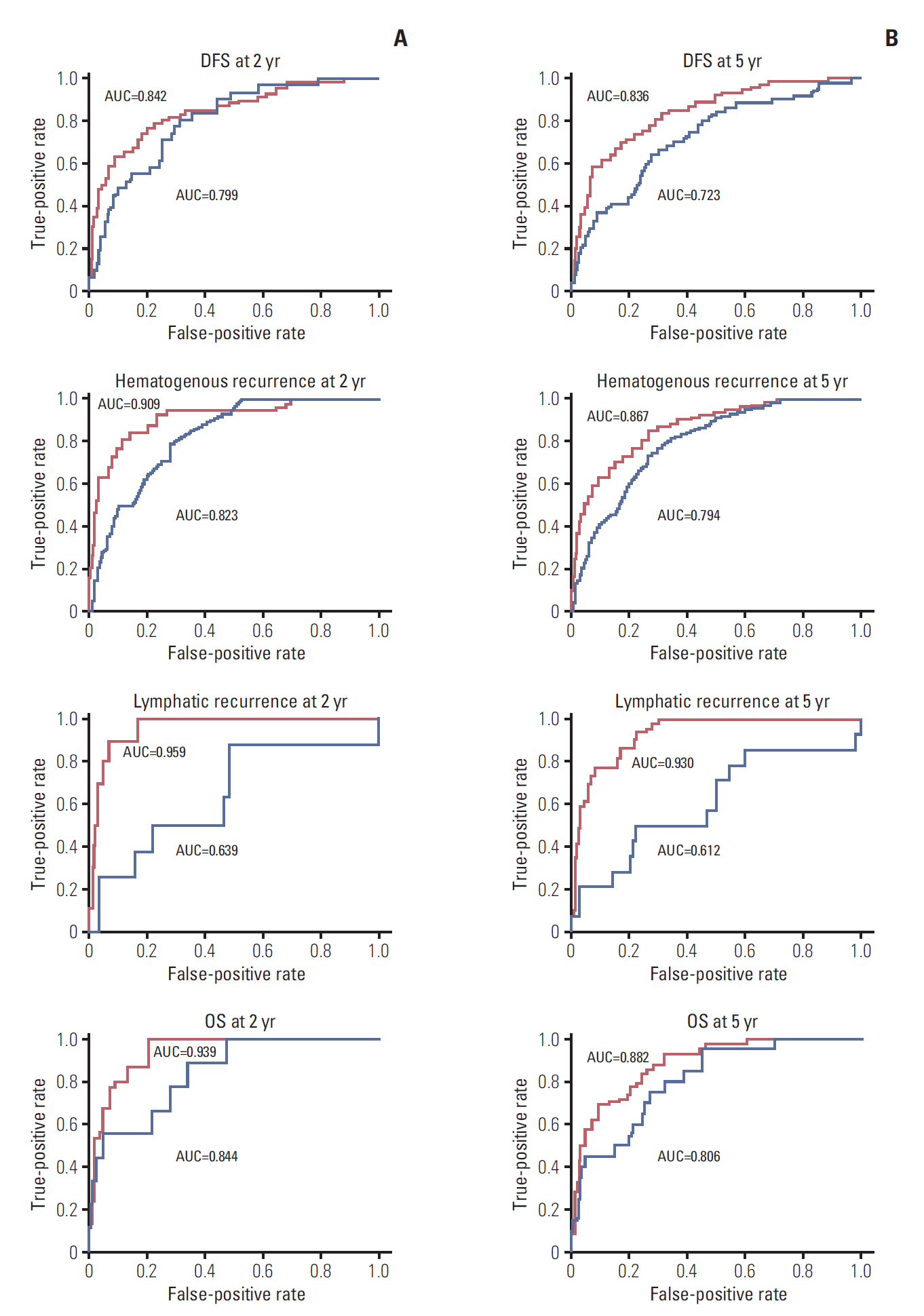
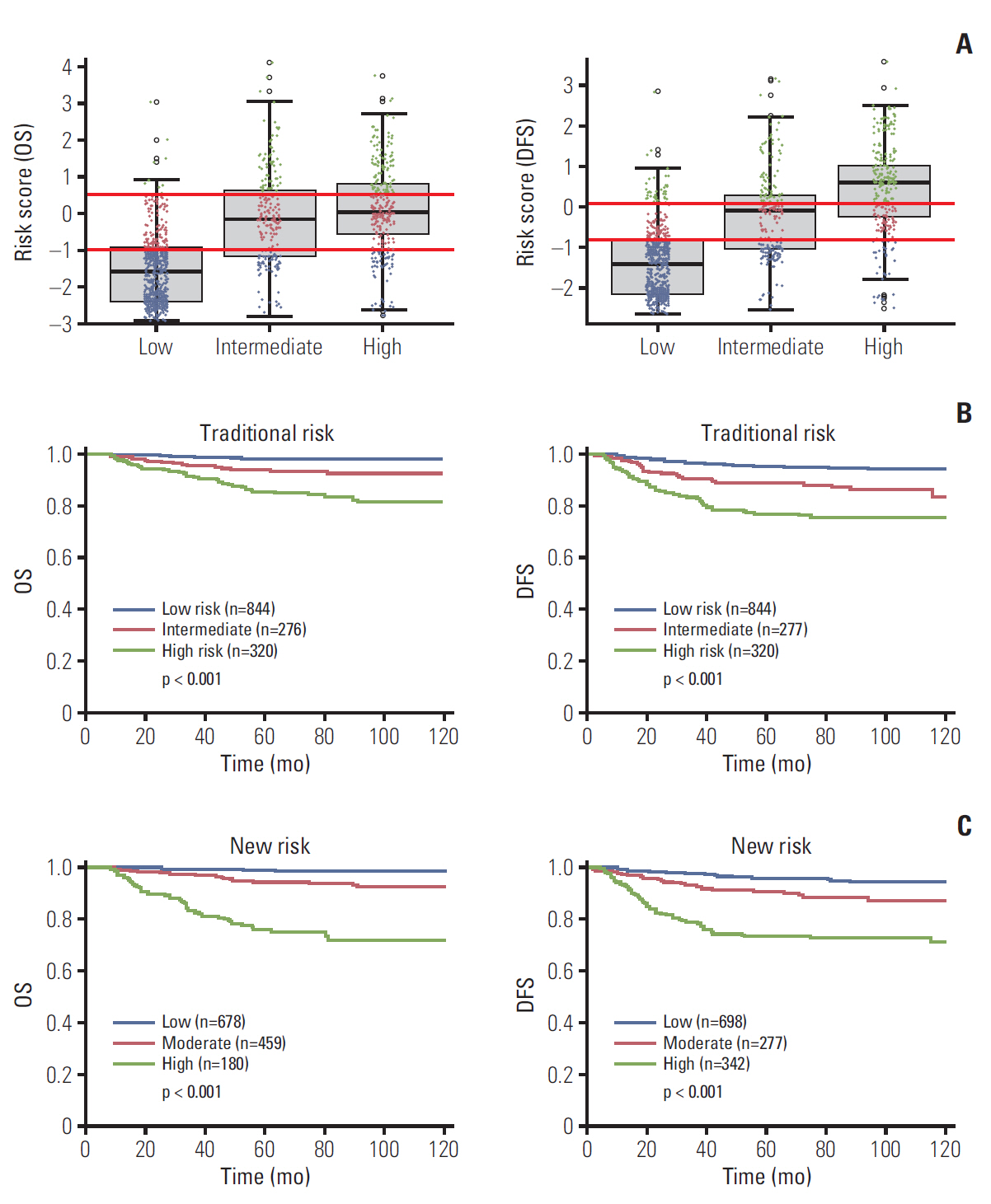
Independent risk factors for OS, DFS, lymphatic recurrence, and hematogenous recurrence were identified for prediction model development. Different combinations of risk factors were shown for each outcome with best predictive value. In train cohort, area under the curve (AUC) at 2 and 5 years were 0.842/0.836 for recurrence, and 0.939/0.882 for OS. When applied to a test cohort, the model also showed accurate prediction result (AUC at 2 and 5 years were 0.799/0.723 for recurrence, and 0.844/0.806 for OS, respectively). The Kaplan-Meier plot by proposed model-classified risk groups showed more distinctive survival differences between each risk group.
Conclusion
We developed prognostic models for OS, DFS, lymphatic and hematogenous recurrence in patients with early-stage cervical cancer. Combining weighted clinicopathologic factors, the proposed model can give more individualized predictions in clinical practice.
Keyword
Figure
Reference
-
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–86.
Article2. Lee WC, Lee SY, Koo YJ, Kim TJ, Hur SY, Hong SR, et al. Establishment of a Korea HPV cohort study. J Gynecol Oncol. 2013; 24:59–65.
Article3. Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990; 38:352–7.
Article4. Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997; 350:535–40.
Article5. Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group study. Gynecol Oncol. 1999; 73:177–83.
Article6. Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000; 18:1606–13.
Article7. Barter JF, Soong SJ, Shingleton HM, Hatch KD, Orr JW Jr. Complications of combined radical hysterectomy-postoperative radiation therapy in women with early stage cervical cancer. Gynecol Oncol. 1989; 32:292–6.
Article8. Lagasse LD, Creasman WT, Shingleton HM, Ford JH, Blessing JA. Results and complications of operative staging in cervical cancer: experience of the Gynecologic Oncology Group. Gynecol Oncol. 1980; 9:90–8.
Article9. Sevin BU, Nadji M, Lampe B, Lu Y, Hilsenbeck S, Koechli OR, et al. Prognostic factors of early stage cervical cancer treated by radical hysterectomy. Cancer. 1995; 76(10 Suppl):1978–86.
Article10. Soisson AP, Soper JT, Clarke-Pearson DL, Berchuck A, Montana G, Creasman WT. Adjuvant radiotherapy following radical hysterectomy for patients with stage IB and IIA cervical cancer. Gynecol Oncol. 1990; 37:390–5.
Article11. Kristensen GB, Abeler VM, Risberg B, Trop C, Bryne M. Tumor size, depth of invasion, and grading of the invasive tumor front are the main prognostic factors in early squamous cell cervical carcinoma. Gynecol Oncol. 1999; 74:245–51.
Article12. Zaino RJ, Ward S, Delgado G, Bundy B, Gore H, Fetter G, et al. Histopathologic predictors of the behavior of surgically treated stage IB squamous cell carcinoma of the cervix. A Gynecologic Oncology Group study. Cancer. 1992; 69:1750–8.
Article13. Tsai CS, Lai CH, Wang CC, Chang JT, Chang TC, Tseng CJ, et al. The prognostic factors for patients with early cervical cancer treated by radical hysterectomy and postoperative radiotherapy. Gynecol Oncol. 1999; 75:328–33.
Article14. Ryu SY, Kim MH, Nam BH, Lee TS, Song ES, Park CY, et al. Intermediate-risk grouping of cervical cancer patients treated with radical hysterectomy: a Korean Gynecologic Oncology Group study. Br J Cancer. 2014; 110:278–85.
Article15. Abu-Rustum NR, Zhou Q, Gomez JD, Alektiar KM, Hensley ML, Soslow RA, et al. A nomogram for predicting overall survival of women with endometrial cancer following primary therapy: toward improving individualized cancer care. Gynecol Oncol. 2010; 116:399–403.
Article16. Ho CM, Chien TY, Huang SH, Wu CJ, Shih BY, Chang SC. Multivariate analysis of the prognostic factors and outcomes in early cervical cancer patients undergoing radical hysterectomy. Gynecol Oncol. 2004; 93:458–64.
Article17. Lai CH, Chang CJ, Huang HJ, Hsueh S, Chao A, Yang JE, et al. Role of human papillomavirus genotype in prognosis of early-stage cervical cancer undergoing primary surgery. J Clin Oncol. 2007; 25:3628–34.
Article18. Yuan CC, Wang PH, Lai CR, Yen MS, Chen CY, Juang CM. Prognosis-predicting system based on factors related to survival of cervical carcinoma. Int J Gynaecol Obstet. 1998; 63:163–7.
Article19. Greenland S, Schwartzbaum JA, Finkle WD. Problems due to small samples and sparse data in conditional logistic regression analysis. Am J Epidemiol. 2000; 151:531–9.
Article20. Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000; 56:337–44.
Article21. Sevin BU, Lu Y, Bloch DA, Nadji M, Koechli OR, Averette HE. Surgically defined prognostic parameters in patients with early cervical carcinoma: a multivariate survival tree analysis. Cancer. 1996; 78:1438–46.
Article22. Smiley LM, Burke TW, Silva EG, Morris M, Gershenson DM, Wharton JT. Prognostic factors in stage IB squamous cervical cancer patients with low risk for recurrence. Obstet Gynecol. 1991; 77:271–5.
Article23. Ishikawa H, Nakanishi T, Inoue T, Kuzuya K. Prognostic factors of adenocarcinoma of the uterine cervix. Gynecol Oncol. 1999; 73:42–6.
Article24. Odell EW, Jani P, Sherriff M, Ahluwalia SM, Hibbert J, Levison DA, et al. The prognostic value of individual histologic grading parameters in small lingual squamous cell carcinomas. The importance of the pattern of invasion. Cancer. 1994; 74:789–94.
Article25. Biewenga P, van der Velden J, Mol BW, Stalpers LJ, Schilthuis MS, van der Steeg JW, et al. Validation of existing prognostic models in patients with early-stage cervical cancer. Gynecol Oncol. 2009; 115:277–84.
Article26. Van de Putte G, Lie AK, Vach W, Baekelandt M, Kristensen GB. Risk grouping in stage IB squamous cell cervical carcinoma. Gynecol Oncol. 2005; 99:106–12.
Article27. Aoki Y, Sasaki M, Watanabe M, Sato T, Tsuneki I, Aida H, et al. High-risk group in node-positive patients with stage IB, IIA, and IIB cervical carcinoma after radical hysterectomy and postoperative pelvic irradiation. Gynecol Oncol. 2000; 77:305–9.
Article28. Lai CH, Hong JH, Hsueh S, Ng KK, Chang TC, Tseng CJ, et al. Preoperative prognostic variables and the impact of postoperative adjuvant therapy on the outcomes of Stage IB or II cervical carcinoma patients with or without pelvic lymph node metastases: an analysis of 891 cases. Cancer. 1999; 85:1537–46.29. Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018; 379:1895–904.
Article30. Benard VB, Watson M, Saraiya M, Harewood R, Townsend JS, Stroup AM, et al. Cervical cancer survival in the United States by race and stage (2001-2009): findings from the CONCORD2 study. Cancer. 2017; 123:5119–37.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impact of diabetes mellitus on oncological outcomes after radical hysterectomy for early stage cervical cancer
- Adjuvant pelvic radiation versus observation in intermediate-risk early-stage cervical cancer patients following primary radical surgery: a propensity score-adjusted analysis
- Who should be offered non-radical surgery for early-stage cervical cancer?
- Parametrial involvement and decreased survival of women with FIGO stage IIIC1 cervical cancer
- Management of patients with intermediate-risk early stage cervical cancer