J Korean Med Sci.
2020 Apr;35(19):e151. 10.3346/jkms.2020.35.e151.
Predicting First-Year Growth in Response to Growth Hormone Treatment in Prepubertal Korean Children with Idiopathic Growth Hormone Deficiency: Analysis of Data from the LG Growth Study Database
- Affiliations
-
- 1Department of Pediatrics, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Pediatrics, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Department of Statistics & Data Monitoring, Life Science R&D, LG Chem, Ltd., Seoul, Korea
- 4Department of Pediatrics, Bucheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 5Department of Pediatrics, Yeouido St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2500632
- DOI: http://doi.org/10.3346/jkms.2020.35.e151
Abstract
- Background
The first-year growth in response to growth hormone (GH) treatment seems to be the most important factor in determining the overall success of GH treatment.
Methods
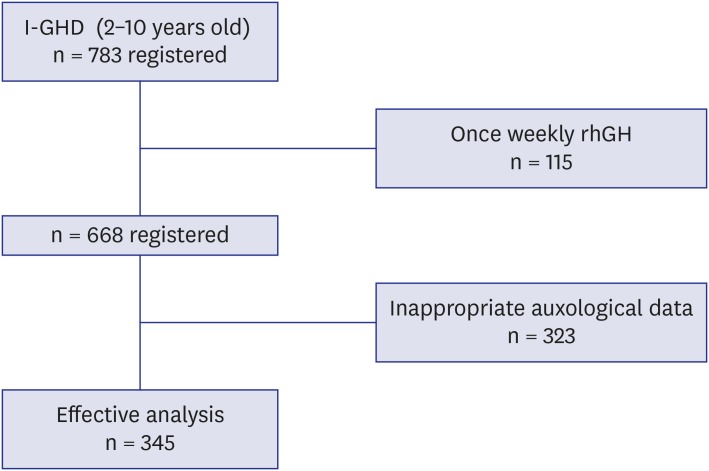
Data from children (n = 345) who were in the LG Growth Study Database were used to develop a model. All subjects had been diagnosed with idiopathic growth hormone deficiency (GHD) and presented in a prepubertal state during the first year of GH treatment.
Results
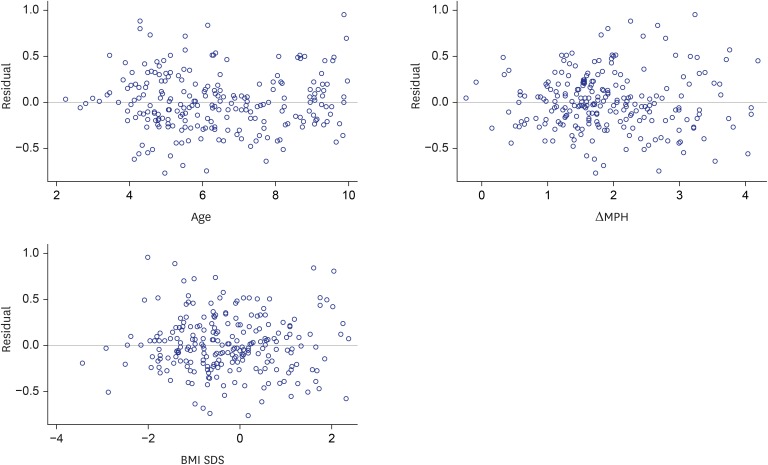
The Δheight standard deviation score (SDS) during 1st year of GH treatment was correlated positively with weight-SDS (β = 0.304, P < 0.001), body mass index (BMI)-SDS (β = 0.443, P < 0.001), paternal height-SDS (β = 0.296, P = 0.001), MPH-SDS (β = 0.421, P < 0.001) and MPH SDS minus baseline height SDS (β = 0.099, P < 0.001) but negatively with chronological age (β = −0.294, P < 0.001), bone age (β = −0.249, P < 0.001). A prediction model of 1st year growth in response to GH treatment in prepubertal Korean children with idiopathic GHD is as follows: Δheight SDS during 1st year of GH treatment = 1.06 − 0.05 × age + 0.09 × (MPH SDS minus baseline height SDS) + 0.05 × BMI SDS. This model explained 19.6% of the variability in the response, with a standard error of 0.31.
Conclusion
The present model to predict first-year response to GH treatment might allow more tailored and personalized GH treatment in Korean prepubertal children with idiopathic GHD.
Keyword
Figure
Reference
-
1. Ranke MB, Wit JM. Growth hormone - past, present and future. Nat Rev Endocrinol. 2018; 14(5):285–300. PMID: 29546874.
Article2. GH Research Society. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. J Clin Endocrinol Metab. 2000; 85(11):3990–3993. PMID: 11095419.3. Reiter EO, Price DA, Wilton P, Albertsson-Wikland K, Ranke MB. Effect of growth hormone (GH) treatment on the near-final height of 1258 patients with idiopathic GH deficiency: analysis of a large international database. J Clin Endocrinol Metab. 2006; 91(6):2047–2054. PMID: 16537676.
Article4. Kang JC, Choi YS, Choi IK, Kim HS, Kim DH. The effect of growth hormone on patients with growth hormone deficiency and idiopathic short stature. Korean J Pediatr. 2004; 47(3):310–318.5. Reinehr T, Bechtold-Dalla Pozza S, Bettendorf M, Doerr HG, Gohlke B, Hauffa BP, et al. Impact of overweight on effectiveness of treatment with human growth hormone in growth hormone deficient children: analysis of German KIGS data. Exp Clin Endocrinol Diabetes. 2011; 119(9):544–548. PMID: 22006181.
Article6. Boguszewski MC, Karlsson H, Wollmann HA, Wilton P, Dahlgren J. Growth hormone treatment in short children born prematurely--data from KIGS. J Clin Endocrinol Metab. 2011; 96(6):1687–1694. PMID: 21430029.
Article7. Ranke MB, Guilbaud O, Lindberg A, Cole T. Prediction of the growth response in children with various growth disorders treated with growth hormone: analyses of data from the Kabi Pharmacia International Growth Study. International Board of the Kabi Pharmacia International Growth Study. Acta Paediatr Suppl. 1993; 82(Suppl 391):82–88.8. Chung S, Yoo JH, Choi JH, Rhie YJ, Chae HW, Kim JH, et al. Design of the long-term observational cohort study with recombinant human growth hormone in Korean children: LG Growth Study. Ann Pediatr Endocrinol Metab. 2018; 23(1):43–50. PMID: 29609449.
Article9. Rhie YJ, Yoo JH, Choi JH, Chae HW, Kim JH, Chung S, et al. Long-term safety and effectiveness of growth hormone therapy in Korean children with growth disorders: 5-year results of LG Growth Study. PLoS One. 2019; 14(5):e0216927. PMID: 31095622.
Article10. Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. 2018; 61(5):135–149. PMID: 29853938.
Article11. Hyun SE, Lee BC, Suh BK, Chung SC, Ko CW, Kim HS, et al. Reference values for serum levels of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in Korean children and adolescents. Clin Biochem. 2012; 45(1-2):16–21. PMID: 22032863.
Article12. Tanner JM, Whitehouse RH, Takaishi M. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children, 1965. II. Arch Dis Child. 1966; 41(220):613–635. PMID: 5927918.
Article13. Weisberg S. Applied Linear Regression. 2nd ed. New York, NY: Wiley and Sons;1985.14. Cook RD, Weisberg S. Residuals and Influence in Regression. New York, NY: Chapman and Hall;1982.15. de Ridder MA, Stijnen T, Hokken-Koelega AC. Prediction of adult height in growth-hormone-treated children with growth hormone deficiency. J Clin Endocrinol Metab. 2007; 92(3):925–931. PMID: 17179199.
Article16. Ranke MB, Lindberg A, Chatelain P, Wilton P, Cutfield W, Albertsson-Wikland K, et al. Derivation and validation of a mathematical model for predicting the response to exogenous recombinant human growth hormone (GH) in prepubertal children with idiopathic GH deficiency. KIGS International Board. Kabi Pharmacia International Growth Study. J Clin Endocrinol Metab. 1999; 84(4):1174–1183. PMID: 10199749.17. Ranke MB, Lindberg A. KIGS International Board. Height at start, first-year growth response and cause of shortness at birth are major determinants of adult height outcomes of short children born small for gestational age and Silver-Russell syndrome treated with growth hormone: analysis of data from KIGS. Horm Res Paediatr. 2010; 74(4):259–266. PMID: 20431273.
Article18. Ranke MB, Lindberg A. Predicting growth in response to growth hormone treatment. Growth Horm IGF Res. 2009; 19(1):1–11. PMID: 18824380.
Article19. Cutfield W, Lindberg A, Albertsson Wikland K, Chatelain P, Ranke MB, Wilton P, et al. Final height in idiopathic growth hormone deficiency: the KIGS experience. Acta Paediatr Suppl. 1999; 88(428):72–75.
Article20. Cohen P, Bright GM, Rogol AD, Kappelgaard AM, Rosenfeld RG. American Norditropin Clinical Trials Group. Effects of dose and gender on the growth and growth factor response to GH in GH-deficient children: implications for efficacy and safety. J Clin Endocrinol Metab. 2002; 87(1):90–98. PMID: 11788629.
Article21. De Muinck Keizer-Schrama S, Rikken B, Hokken-Koelega A, Wit JM, Drop S. The Dutch Growth Hormone Working Group. Comparative effect of two doses of growth hormone for growth hormone deficiency. Arch Dis Child. 1994; 71(1):12–18. PMID: 8067786.22. Kriström B, Jansson C, Rosberg S, Albertsson-Wikland K. Swedish Study Group for Growth Hormone Treatment. Growth response to growth hormone (GH) treatment relates to serum insulin-like growth factor I (IGF-I) and IGF-binding protein-3 in short children with various GH secretion capacities. J Clin Endocrinol Metab. 1997; 82(9):2889–2898. PMID: 9284715.23. Ranke MB, Schweizer R, Elmlinger MW, Weber K, Binder G, Schwarze CP, et al. Significance of basal IGF-I, IGFBP-3 and IGFBP-2 measurements in the diagnostics of short stature in children. Horm Res. 2000; 54(2):60–68. PMID: 11251368.
Article24. Blum WF, Ranke MB, Kietzmann K, Gauggel E, Zeisel HJ, Bierich JR. A specific radioimmunoassay for the growth hormone (GH)-dependent somatomedin-binding protein: its use for diagnosis of GH deficiency. J Clin Endocrinol Metab. 1990; 70(5):1292–1298. PMID: 1692331.
Article25. Chanson P, Arnoux A, Mavromati M, Brailly-Tabard S, Massart C, Young J, et al. Reference values for IGF-I serum concentrations: comparison of six immunoassays. J Clin Endocrinol Metab. 2016; 101(9):3450–3458. PMID: 27167056.
Article26. Ranke MB, Schweizer R, Lindberg A, Price DA, Reiter EO, Albertsson-Wikland K, et al. Insulin-like growth factors as diagnostic tools in growth hormone deficiency during childhood and adolescence: the KIGS experience. Horm Res. 2004; 62(Suppl 1):17–25.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical effects of yeast derived recombinant methionyl growth hormone in growth hormone deficiency
- Effect of yeast-derived methionyl recombinant growth hormone on growth hormone deficient dwarf
- Quantification of human urinary growth hormone and its clinical significance in the diagnosis of growth hormone deficiency
- Response to growth hormone according to provocation test results in idiopathic short stature and idiopathic growth hormone deficiency
- Short Stature and Growth Hormone Therapy