Int J Stem Cells.
2020 Mar;13(1):65-79. 10.15283/ijsc19069.
Molecular Characterization of Embryonic Stem Cell-Derived Cardiac Neural Crest-Like Cells Revealed a Spatiotemporal Expression of an Mlc-3 Isoform
- Affiliations
-
- 1Institute of Human Genetics, Department of Human Molecular Genetics, Heidelberg University Hospital, Heidelberg, Germany
- 2Institute for Biological Interfaces 1, Karlsruhe Institute of Technology (KIT), Eggenstein-Leopoldshafen, Germany
- 3Medical Faculty Mannheim, Mannheim, Germany
- KMID: 2500567
- DOI: http://doi.org/10.15283/ijsc19069
Abstract
- Background and Objectives
Pluripotent embryonic stem (ES) cells represent a perfect model system for the investigation of early developmental processes. Besides their differentiation into derivatives of the three primary germ layers, they can also be differentiated into derivatives of the ‘fourth’ germ layer, the neural crest (NC). Due to its multipotency, extensive migration and outstanding capacity to generate a remarkable number of different cell types, the NC plays a key role in early developmental processes. Cardiac neural crest (CNC) cells are a subpopulation of the NC, which are of crucial importance for precise cardiovascular and pharyngeal glands’ development. CNC-associated malformations are rare, but always severe and life-threatening. Appropriate cell models could help to unravel underlying pathomechanisms and to develop new therapeutic options for relevant heart malformations.
Methods
Murine ES cells were differentiated according to a mesodermal-lineage promoting protocol. Expression profiles of ES cell-derived progeny at various differentiation stages were investigated on transcript and protein level.
Results
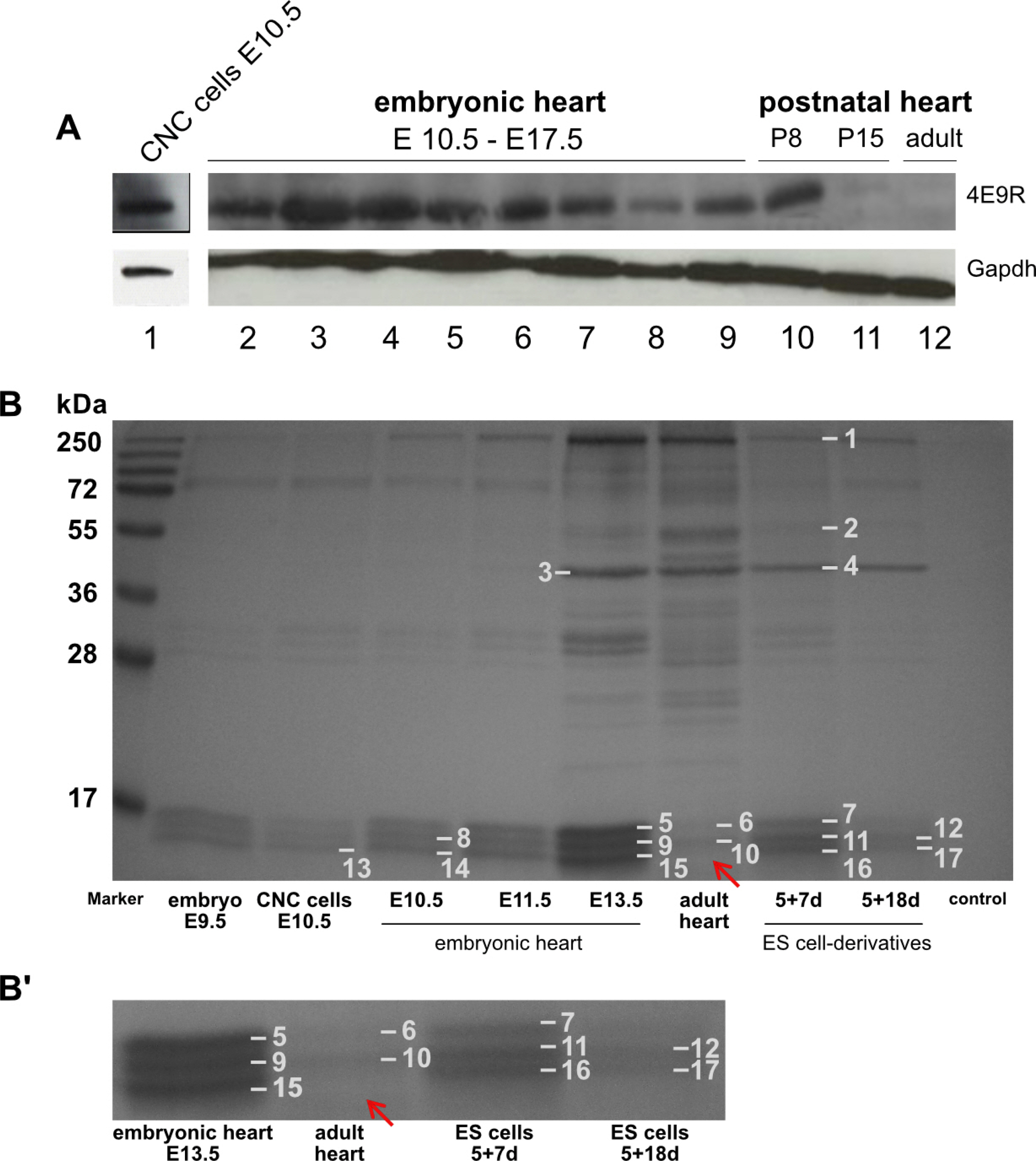
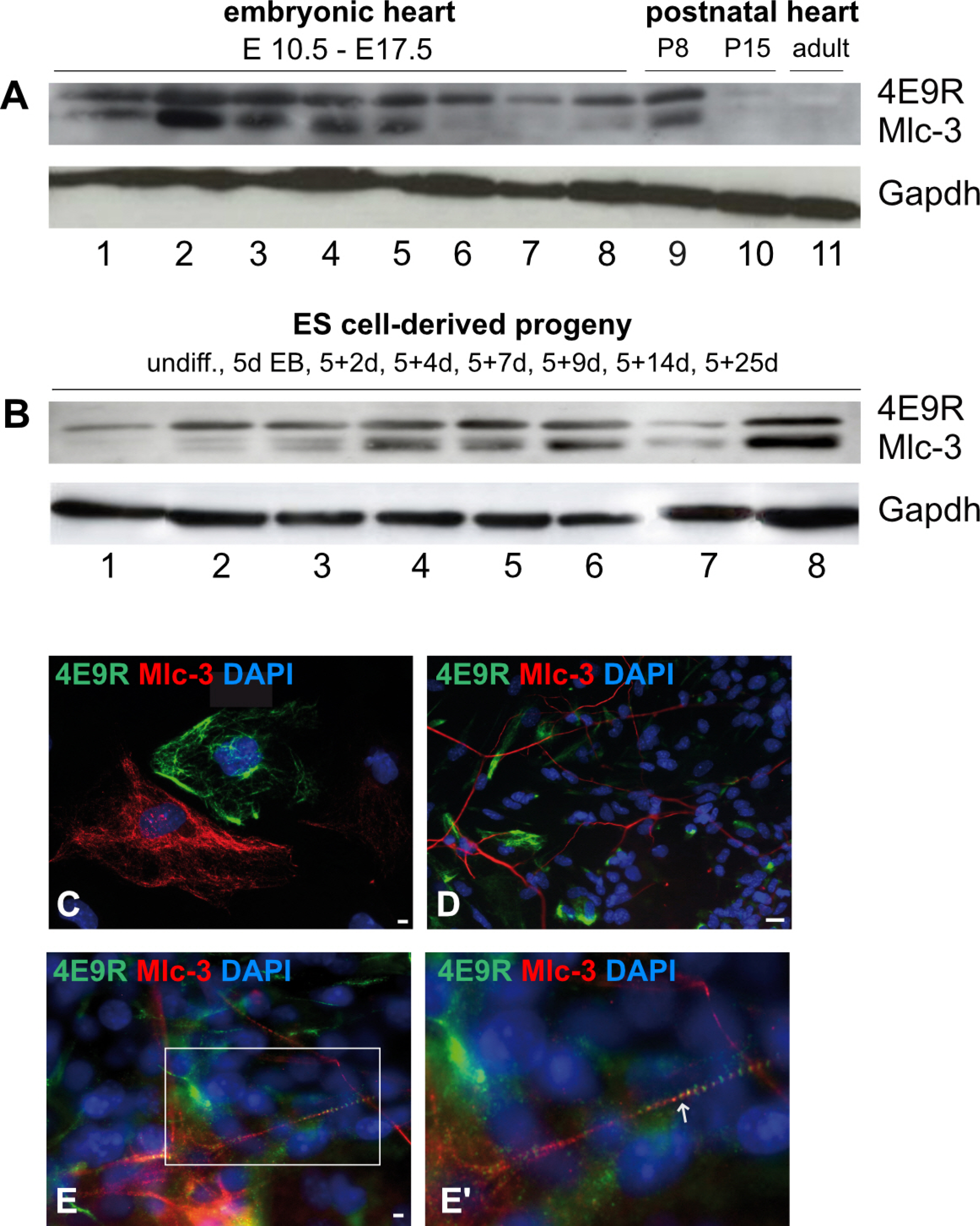
Comparative expression profiling of murine ES cell multilineage progeny versus undifferentiated ES cells confirmed differentiation into known cell derivatives of the three primary germ layers and provided evidence that ES cells have the capacity to differentiate into NC/CNC-like cells. Applying the NC/CNC cell-specific marker, 4E9R, an unambiguous identification of ES cell-derived NC/CNC-like cells was achieved.
Conclusions
Our findings will facilitate the establishment of an ES cell-derived CNC cell model for the investigation of molecular pathways during cardiac development in health and disease.
Keyword
Figure
Cited by 1 articles
-
Direct Conversion to Achieve Glial Cell Fates: Oligodendrocytes and Schwann Cells
Wonjin Yun, Yong Jun Kim, Gabsang Lee
Int J Stem Cells. 2022;15(1):14-25. doi: 10.15283/ijsc22008.
Reference
-
References
1. Evans MJ, Kaufman MH. 1981; Establishment in culture of pluripotential cells from mouse embryos. Nature. 292:154–156. DOI: 10.1038/292154a0. PMID: 7242681.
Article2. Martin GR. 1981; Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 78:7634–7638. DOI: 10.1073/pnas.78.12.7634. PMID: 6950406. PMCID: PMC349323.
Article3. Wobus AM. 2001; Potential of embryonic stem cells. Mol Aspects Med. 22:149–164. DOI: 10.1016/S0098-2997(01)00006-1.
Article4. Prelle K, Zink N, Wolf E. 2002; Pluripotent stem cells--model of embryonic development, tool for gene targeting, and basis of cell therapy. Anat Histol Embryol. 31:169–186. DOI: 10.1046/j.1439-0264.2002.00388.x. PMID: 12479360.
Article5. Boheler KR, Czyz J, Tweedie D, Yang HT, Anisimov SV, Wobus AM. 2002; Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res. 91:189–201. DOI: 10.1161/01.RES.0000027865.61704.32. PMID: 12169644.
Article6. Rolletschek A, Schroeder IS, Schulz H, Hummel O, Huebner N, Wobus AM. 2010; Characterization of mouse embryonic stem cell differentiation into the pancreatic lineage in vitro by transcriptional profiling, quantitative RT-PCR and immunocytochemistry. Int J Dev Biol. 54:41–54. DOI: 10.1387/ijdb.082694ar. PMID: 19876843.
Article7. Le Douarin N. 1982. The neural crest. Cambridge University Press;Cambridge: p. xi-259.8. Gilbert SF. Gilbert SF, editor. 2000. Developmental biology. The neural crest. 6th ed. Sinauer Associates;Sunderland:9. Kirby ML, Gale TF, Stewart DE. 1983; Neural crest cells contribute to normal aorticopulmonary septation. Science. 220:1059–1061. DOI: 10.1126/science.6844926. PMID: 6844926.
Article10. Kirby ML, Waldo KL. 1995; Neural crest and cardiovascular patterning. Circ Res. 77:211–215. DOI: 10.1161/01.RES.77.2.211. PMID: 7614707.
Article11. Miyagawa-Tomita S, Arima Y, Kurihara H. Nakanishi T, Markwald RR, Baldwin HS, Keller BB, Srivastava D, Yamagishi H, editors. 2016; Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology. The "Cardiac Neural Crest" concept revisited. Springer;Tokyo: 227–232. DOI: 10.1007/978-4-431-54628-3_30. PMID: 29787146.12. YamagishiBesson WT 3rd, Kirby ML, Van Mierop LH, Teabeaut JR 2nd. 1986; Effects of the size of lesions of the cardiac neural crest at various embryonic ages on incidence and type of cardiac defects. Circulation. 73:360–364. DOI: 10.1161/01.CIR.73.2.360. PMID: 3943168.
Article13. Bockman DE, Redmond ME, Waldo K, Davis H, Kirby ML. 1987; Effect of neural crest ablation on development of the heart and arch arteries in the chick. Am J Anat. 180:332–341. DOI: 10.1002/aja.1001800403. PMID: 3425561.
Article14. Creazzo TL, Brotto MA, Burch J. 1997; Excitation-contraction coupling in the day 15 embryonic chick heart with persistent truncus arteriosus. Pediatr Res. 42:731–737. DOI: 10.1203/00006450-199712000-00002. PMID: 9396550.
Article15. Nosek TM, Fogaça RT, Hatcher CJ, Brotto MA, Godt RE. 1997; Effect of cardiac neural crest ablation on contractile force and calcium uptake and release in chick heart. Am J Physiol. 273:H1464–H1471. DOI: 10.1152/ajpheart.1997.273.3.H1464. PMID: 9321838.
Article16. Conway SJ, Henderson DJ, Kirby ML, Anderson RH, Copp AJ. 1997; Development of a lethal congenital heart defect in the splotch (Pax3) mutant mouse. Cardiovasc Res. 36:163–173. DOI: 10.1016/S0008-6363(97)00172-7. PMID: 9463628.
Article17. Conway SJ, Godt RE, Hatcher CJ, Leatherbury L, Zolotouchnikov VV, Brotto MA, Copp AJ, Kirby ML, Creazzo TL. 1997; Neural crest is involved in development of abnormal myocardial function. J Mol Cell Cardiol. 29:2675–2685. DOI: 10.1006/jmcc.1997.0499. PMID: 9344762.
Article18. Epstein JA, Li J, Lang D, Chen F, Brown CB, Jin F, Lu MM, Thomas M, Liu E, Wessels A, Lo CW. 2000; Migration of cardiac neural crest cells in Splotch embryos. Development. 127:1869–1878. PMID: 10751175.
Article19. Li J, Liu KC, Jin F, Lu MM, Epstein JA. 1999; Transgenic rescue of congenital heart disease and spina bifida in Splotch mice. Development. 126:2495–2503. PMID: 10226008.
Article20. Keyte A, Hutson MR. 2012; The neural crest in cardiac congenital anomalies. Differentiation. 84:25–40. DOI: 10.1016/j.diff.2012.04.005. PMID: 22595346. PMCID: PMC3389200.
Article21. Brand T. 2003; Heart development: molecular insights into cardiac specification and early morphogenesis. Dev Biol. 258:1–19. DOI: 10.1016/S0012-1606(03)00112-X. PMID: 12781678.
Article22. Kubota Y, Morita T, Ito K. 1996; New monoclonal antibody (4E9R) identifies mouse neural crest cells. Developmental Dynamics. 206:368–378. DOI: 10.1002/(SICI)1097-0177(199608)206:4<368::AID-AJA3>3.0.CO;2-G. PMID: 8853986.
Article23. Youn YH, Feng J, Tessarollo L, Ito K, Sieber-Blum M. 2003; Neural crest stem cell and cardiac endothelium defects in the TrkC null mouse. Mol Cell Neurosci. 24:160–170. DOI: 10.1016/S1044-7431(03)00125-8. PMID: 14550777.
Article24. Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. 1993; Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 90:8424–8428. DOI: 10.1073/pnas.90.18.8424. PMID: 8378314. PMCID: PMC47369.
Article25. Wobus AM, Guan K, Yang HT, Boheler KR. 2002; Embryonic stem cells as a model to study cardiac, skeletal muscle, and vascular smooth muscle cell differentiation. Methods Mol Biol. 185:127–156. DOI: 10.1385/1-59259-241-4:127. PMID: 11768985.
Article26. Hoffmann S, Schmitteckert S, Griesbeck A, Preiss H, Sumer S, Rolletschek A, Granzow M, Eckstein V, Niesler B, Rappold GA. 2017; Comparative expression analysis of Shox2-deficient embryonic stem cell-derived sinoatrial node-like cells. Stem Cell Res. 21:51–57. DOI: 10.1016/j.scr.2017.03.018. PMID: 28390247.
Article27. Schroeder IS, Rolletschek A, Blyszczuk P, Kania G, Wobus AM. 2006; Differentiation of mouse embryonic stem cells to insulin-producing cells. Nat Protoc. 1:495–507. DOI: 10.1038/nprot.2006.71. PMID: 17406275.
Article28. Wiese C, Nikolova T, Zahanich I, Sulzbacher S, Fuchs J, Yamanaka S, Graf E, Ravens U, Boheler KR, Wobus AM. 2011; Differentiation induction of mouse embryonic stem cells into sinus node-like cells by suramin. Int J Cardiol. 147:95–111. DOI: 10.1016/j.ijcard.2009.08.021. PMID: 19775764. PMCID: PMC4751033.
Article29. Maltsev VA, Wobus AM, Rohwedel J, Bader M, Hescheler J. 1994; Cardiomyocytes differentiated in vitro from embryonic stem cells developmentally express cardiac-specific genes and ionic currents. Circ Res. 75:233–244. DOI: 10.1161/01.RES.75.2.233. PMID: 8033337.
Article30. Snider P, Olaopa M, Firulli AB, Conway SJ. 2007; Cardiovascular development and the colonizing cardiac neural crest lineage. ScientificWorldJournal. 7:1090–1113. DOI: 10.1100/tsw.2007.189. PMID: 17619792. PMCID: PMC2613651.
Article31. Liu JA, Cheung M. 2016; Neural crest stem cells and their potential therapeutic applications. Dev Biol. 419:199–216. DOI: 10.1016/j.ydbio.2016.09.006. PMID: 27640086.
Article32. Kawasaki T, Bekku Y, Suto F, Kitsukawa T, Taniguchi M, Nagatsu I, Nagatsu T, Itoh K, Yagi T, Fujisawa H. 2002; Requirement of neuropilin 1-mediated Sema3A signals in patterning of the sympathetic nervous system. Development. 129:671–680. PMID: 11830568.
Article33. Kubota Y, Ito K. 2000; Chemotactic migration of mesencephalic neural crest cells in the mouse. Dev Dyn. 217:170–179. DOI: 10.1002/(SICI)1097-0177(200002)217:2<170::AID-DVDY4>3.0.CO;2-9. PMID: 10706141.
Article34. Labosky PA, Kaestner KH. 1998; The winged helix transcription factor Hfh2 is expressed in neural crest and spinal cord during mouse development. Mech Dev. 76:185–190. DOI: 10.1016/S0925-4773(98)00105-1. PMID: 9767163.
Article35. Cavanaugh AM, Huang J, Chen JN. 2015; Two developmentally distinct populations of neural crest cells contribute to the zebrafish heart. Dev Biol. 404:103–112. DOI: 10.1016/j.ydbio.2015.06.002. PMID: 26086691. PMCID: PMC4515179.
Article36. Li YX, Zdanowicz M, Young L, Kumiski D, Leatherbury L, Kirby ML. 2003; Cardiac neural crest in zebrafish embryos contributes to myocardial cell lineage and early heart function. Dev Dyn. 226:540–550. DOI: 10.1002/dvdy.10264. PMID: 12619138.
Article37. Sato M, Yost HJ. 2003; Cardiac neural crest contributes to cardiomyogenesis in zebrafish. Dev Biol. 257:127–139. DOI: 10.1016/S0012-1606(03)00037-X. PMID: 12710962.
Article38. Tamura Y, Matsumura K, Sano M, Tabata H, Kimura K, Ieda M, Arai T, Ohno Y, Kanazawa H, Yuasa S, Kaneda R, Makino S, Nakajima K, Okano H, Fukuda K. 2011; Neural crest-derived stem cells migrate and differentiate into cardiomyocytes after myocardial infarction. Arterioscler Thromb Vasc Biol. 31:582–589. DOI: 10.1161/ATVBAHA.110.214726. PMID: 21212399.
Article39. Tomita Y, Matsumura K, Wakamatsu Y, Matsuzaki Y, Shibuya I, Kawaguchi H, Ieda M, Kanakubo S, Shimazaki T, Ogawa S, Osumi N, Okano H, Fukuda K. 2005; Cardiac neural crest cells contribute to the dormant multipotent stem cell in the mammalian heart. J Cell Biol. 170:1135–1146. DOI: 10.1083/jcb.200504061. PMID: 16186259. PMCID: PMC2171522.
Article40. Hatzistergos KE, Takeuchi LM, Saur D, Seidler B, Dymecki SM, Mai JJ, White IA, Balkan W, Kanashiro-Takeuchi RM, Schally AV, Hare JM. 2015; cKit+ cardiac progenitors of neural crest origin. Proc Natl Acad Sci U S A. 112:13051–13056. DOI: 10.1073/pnas.1517201112. PMID: 26438843. PMCID: PMC4620867.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Modification of Pluripotency and Neural Crest-Related Genes' expression in Murine Skin-Derived Precursor Cells by Leukemia Inhibitory Factor (LIF)
- Novel Technique for Inducing Neural Crest Fate in Embryonic Stem Cells (Stem Cells 2009;27:2896-2905)
- Regulation of Neural Stem Cell Fate by Natural Products
- Sox9 regulates development of neural crest and otic placode in a time- and dose-dependent fashion
- Current Concepts of Stem Cell Therapy