Cancer Res Treat.
2020 Apr;52(2):455-468. 10.4143/crt.2019.271.
Validation of the 8th Edition of the American Joint Committee onCancer Staging System for Gallbladder Cancer and Implications forthe Follow-up of Patients without Node Dissection
- Affiliations
-
- 1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Pathology, Kyung Hee University Hospital at Gangdong, Seoul, Korea
- 3Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2500331
- DOI: http://doi.org/10.4143/crt.2019.271
Abstract
- Purpose
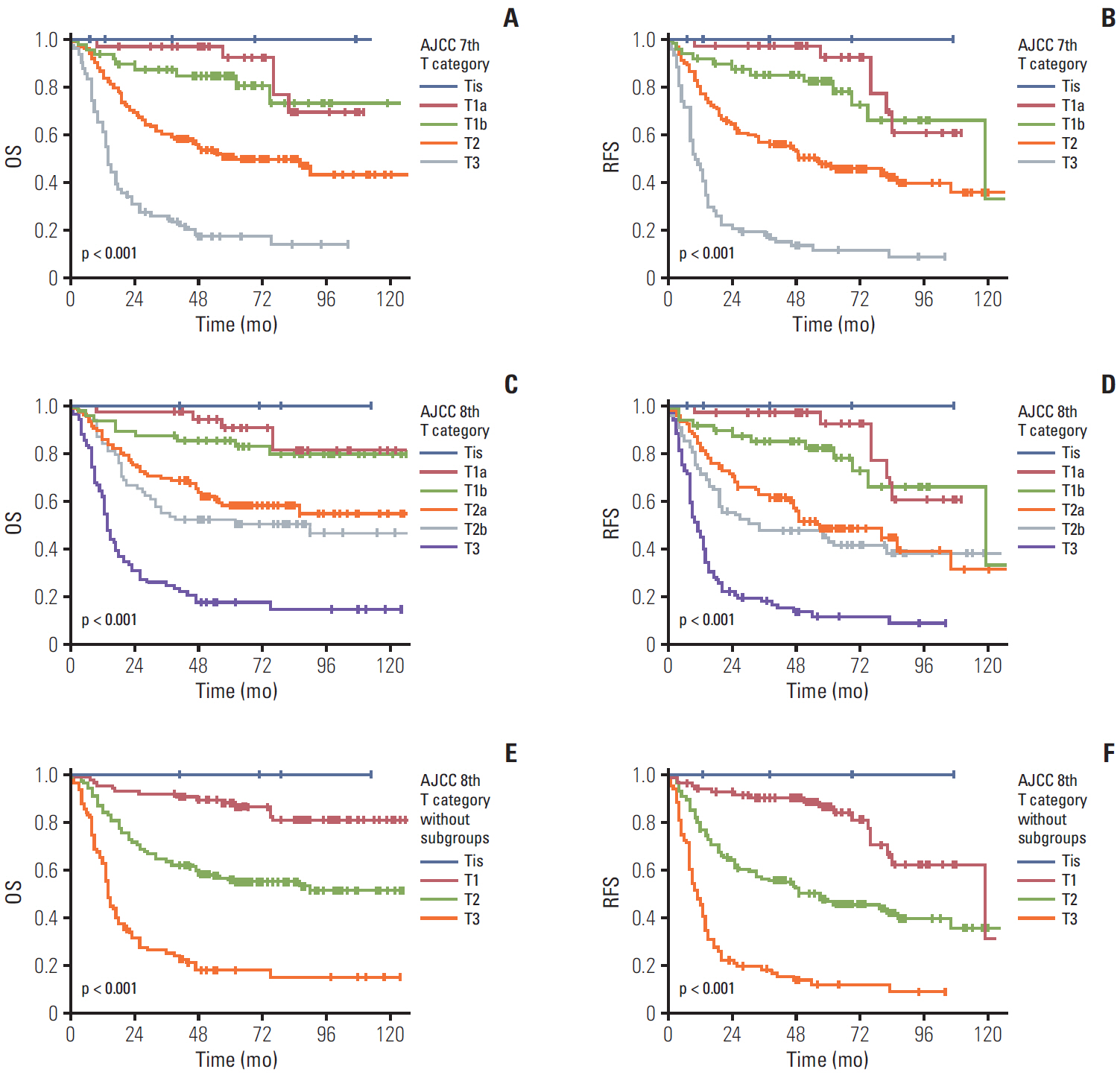
The 8th edition of gallbladder cancer staging in the American Joint Committee on Cancer (AJCC) staging system changed the T and N categories.
Materials and Methods
In order to validate the new staging system, a total of 348 surgically resected gallbladder cancers were grouped based on the 8th edition of the T and N categories and compared with patients’ survival.
Results
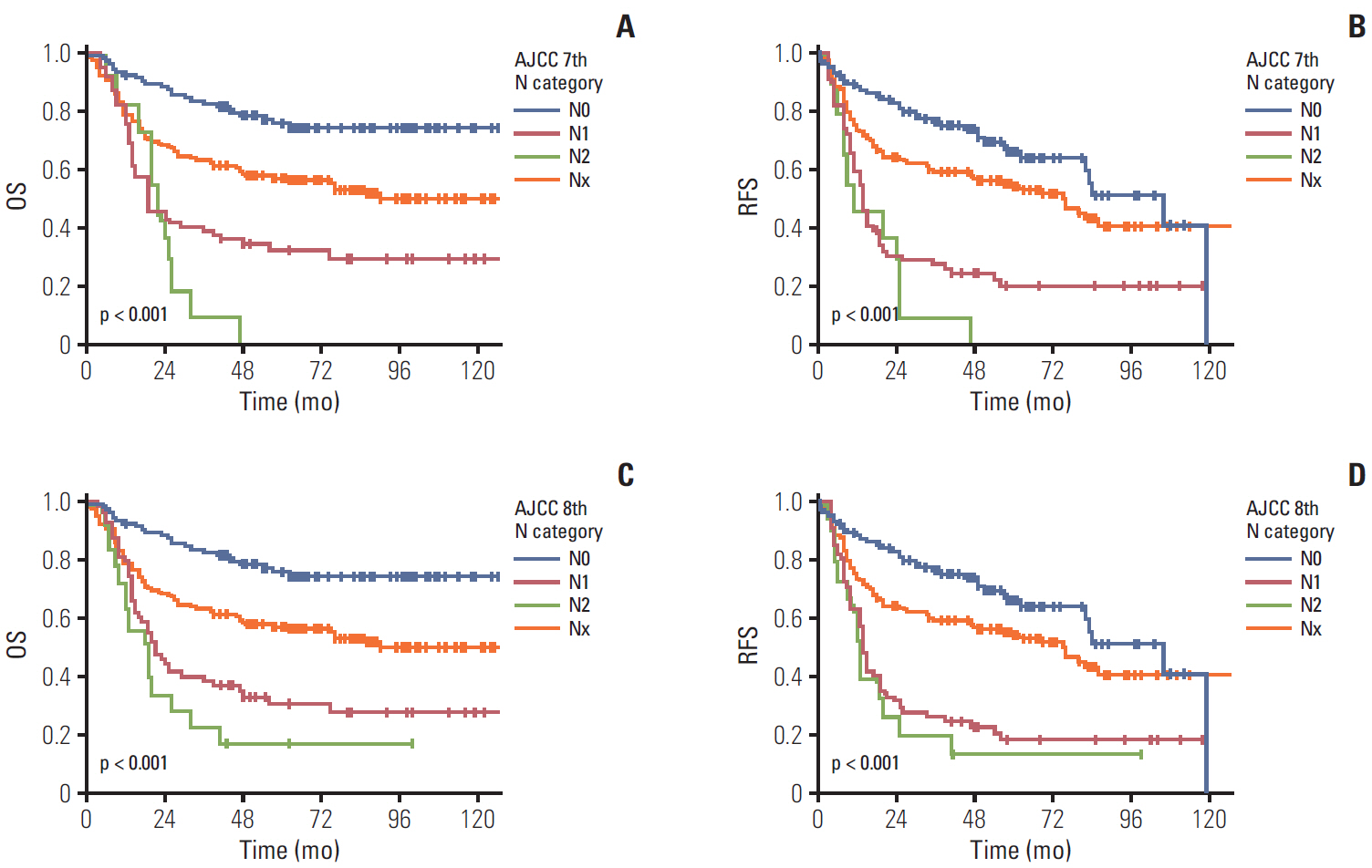
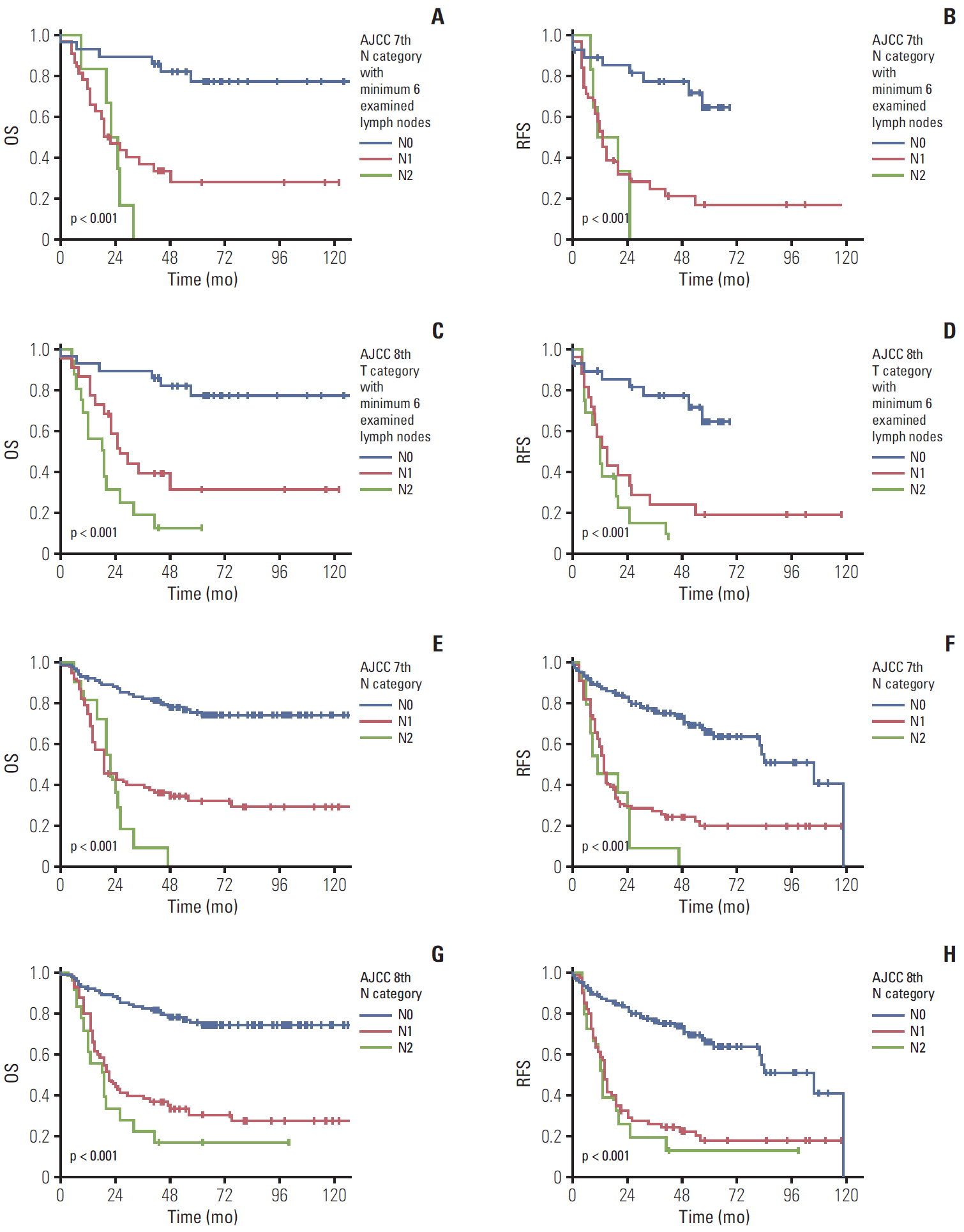
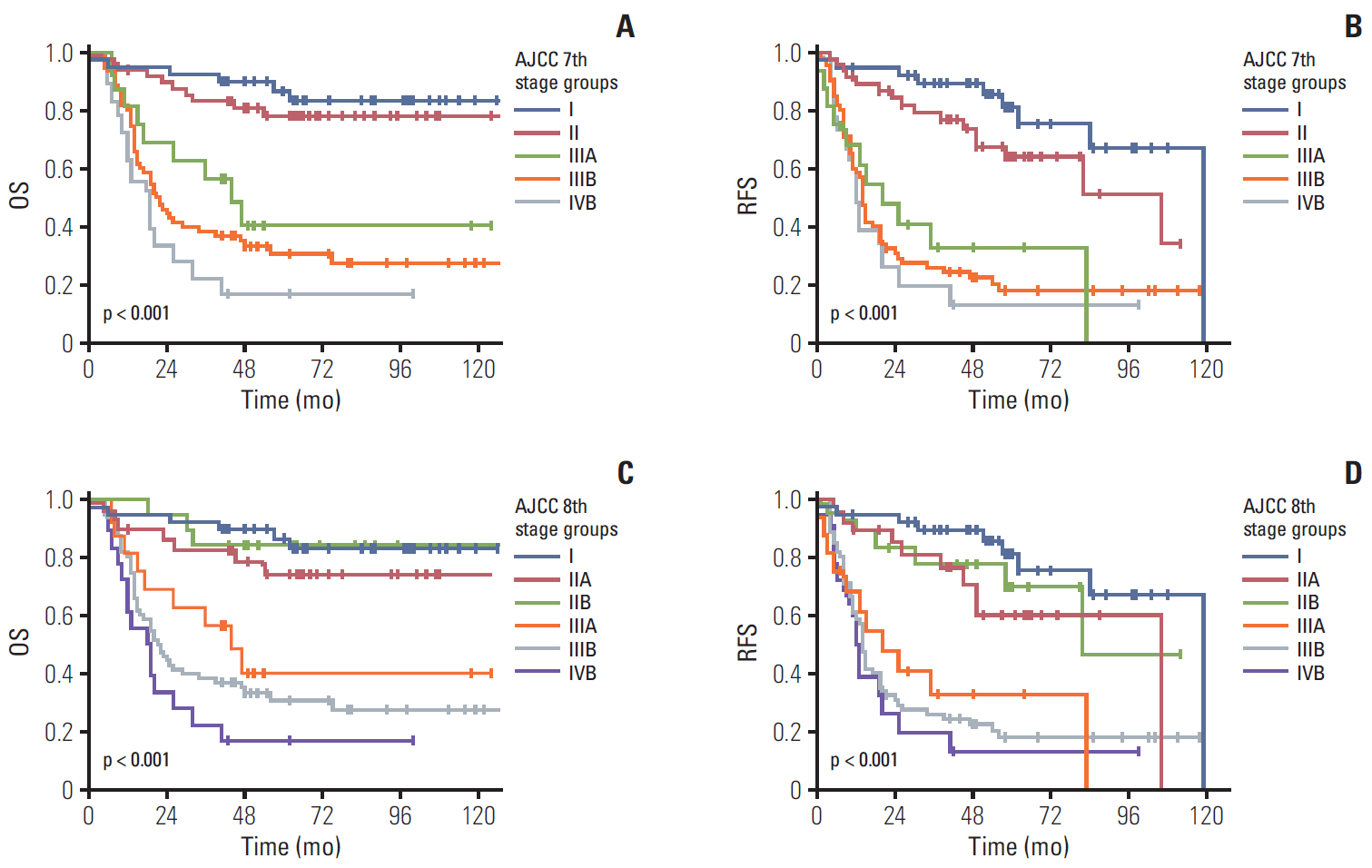
Significant differences were noted between T1b-T2a (p=0.003) and T2b-T3 (p < 0.001) tumors, but not between Tis-T1a, T1a-T1b, and T2a-T2b tumors. However, significant survival differences were observed both by the overall and pair-wise (T1-T2, T2-T3) comparisons (all, p < 0.001) without dividing T1/T2 subcategories. When cases with ! 6 examined lymph nodes were evaluated, significant survival differences were observed among the entire comparison (p < 0.001) and pair-wise comparisons of N0-N1 (p=0.001) and N1-N2 (p=0.039) lesions. When cases without nodal dissection (NX) were additionally compared, significant survival differences were observed between patients with N0-NX (p=0.001) and NX-N1 (p < 0.001) lesions.
Conclusion
The T category in the 8th edition of the AJCC staging system did not completely stratify the prognosis of patients with gallbladder cancer. Modification by eliminating T subcategories can better stratify the prognosis. In contrast, the N category clearly determines patients’ survival with ! 6 examined lymph nodes. The survival time in patients of gallbladder cancers without nodal dissection is between N0 and N1 cases. Therefore, close postoperative followed up is recommended for those patients.
Keyword
Figure
Cited by 1 articles
-
High Systemic Inflammation Response Index (SIRI) Indicates Poor Outcome in Gallbladder Cancer Patients with Surgical Resection: A Single Institution Experience in China
Lejia Sun, Wenmo Hu, Meixi Liu, Yang Chen, Bao Jin, Haifeng Xu, Shunda Du, Yiyao Xu, Haitao Zhao, Xin Lu, Xinting Sang, Shouxian Zhong, Huayu Yang, Yilei Mao
Cancer Res Treat. 2020;52(4):1199-1210. doi: 10.4143/crt.2020.303.
Reference
-
References
1. Vijayakumar A, Vijayakumar A, Patil V, Mallikarjuna MN, Shivaswamy BS. Early diagnosis of gallbladder carcinoma: an algorithm approach. ISRN Radiol. 2013; 2013:239424.
Article2. Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat. 2017; 49:292–305.
Article3. Ahn Y, Park CS, Hwang S, Jang HJ, Choi KM, Lee SG. Incidental gallbladder cancer after routine cholecystectomy: when should we suspect it preoperatively and what are predictors of patient survival? Ann Surg Treat Res. 2016; 90:131–8.
Article4. Muszynska C, Lundgren L, Lindell G, Andersson R, Nilsson J, Sandstrom P, et al. Predictors of incidental gallbladder cancer in patients undergoing cholecystectomy for benign gallbladder disease: Results from a population-based gallstone surgery registry. Surgery. 2017; 162:256–63.
Article5. Elmasry M, Lindop D, Dunne DF, Malik H, Poston GJ, Fenwick SW. The risk of malignancy in ultrasound detected gallbladder polyps: a systematic review. Int J Surg. 2016; 33 Pt A:28–35.
Article6. Ethun CG, Postlewait LM, Le N, Pawlik TM, Buettner S, Poultsides G, et al. A novel pathology-based preoperative risk score to predict locoregional residual and distant disease and survival for incidental gallbladder cancer: a 10-institution study from the U.S. Extrahepatic Biliary Malignancy Consortium. Ann Surg Oncol. 2017; 24:1343–50.
Article7. Hawkins WG, DeMatteo RP, Jarnagin WR, Ben-Porat L, Blumgart LH, Fong Y. Jaundice predicts advanced disease and early mortality in patients with gallbladder cancer. Ann Surg Oncol. 2004; 11:310–5.
Article8. American Cancer Society. Cancer facts and figures 2017. Atlanta, GA: American Cancer Society;2017.9. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washingotn MK, et al. AJCC cancer staging manual. 8th ed. New York: Springer;2017.10. Shindoh J, de Aretxabala X, Aloia TA, Roa JC, Roa I, Zimmitti G, et al. Tumor location is a strong predictor of tumor progression and survival in T2 gallbladder cancer: an international multicenter study. Ann Surg. 2015; 261:733–9.11. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer;2009.12. Amini N, Spolverato G, Kim Y, Gupta R, Margonis GA, Ejaz A, et al. Lymph node status after resection for gallbladder adenocarcinoma: prognostic implications of different nodal staging/scoring systems. J Surg Oncol. 2015; 111:299–305.
Article13. Lee SE, Kim KS, Kim WB, Kim IG, Nah YW, Ryu DH, et al. Practical guidelines for the surgical treatment of gallbladder cancer. J Korean Med Sci. 2014; 29:1333–40.
Article14. Wakai T, Shirai Y, Sakata J, Nagahashi M, Ajioka Y, Hatakeyama K. Mode of hepatic spread from gallbladder carcinoma: an immunohistochemical analysis of 42 hepatectomized specimens. Am J Surg Pathol. 2010; 34:65–74.
Article15. Shirai Y, Tsukada K, Ohtani T, Watanabe H, Hatakeyama K. Hepatic metastases from carcinoma of the gallbladder. Cancer. 1995; 75:2063–8.
Article16. Endo I, Shimada H, Takimoto A, Fujii Y, Miura Y, Sugita M, et al. Microscopic liver metastasis: prognostic factor for patients with pT2 gallbladder carcinoma. World J Surg. 2004; 28:692–6.
Article17. Lindberg MR. Diagnostic pathology: normal histology. 2nd ed. Philadelphia, PA: Elsevier;2017.18. Kim Y, Amini N, Wilson A, Margonis GA, Ethun CG, Poultsides G, et al. Impact of chemotherapy and external-beam radiation therapy on outcomes among patients with resected gallbladder cancer: a multi-institutional analysis. Ann Surg Oncol. 2016; 23:2998–3008.
Article19. Kasumova GG, Tabatabaie O, Najarian RM, Callery MP, Ng SC, Bullock AJ, et al. Surgical management of gallbladder cancer: simple versus extended cholecystectomy and the role of adjuvant therapy. Ann Surg. 2017; 266:625–31.20. Shimada H, Endo I, Fujii Y, Kamiya N, Masunari H, Kunihiro O, et al. Appraisal of surgical resection of gallbladder cancer with special reference to lymph node dissection. Langenbecks Arch Surg. 2000; 385:509–14.
Article21. Sakata J, Shirai Y, Wakai T, Ajioka Y, Hatakeyama K. Number of positive lymph nodes independently determines the prognosis after resection in patients with gallbladder carcinoma. Ann Surg Oncol. 2010; 17:1831–40.
Article22. Endo I, Shimada H, Tanabe M, Fujii Y, Takeda K, Morioka D, et al. Prognostic significance of the number of positive lymph nodes in gallbladder cancer. J Gastrointest Surg. 2006; 10:999–1007.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Assessment of the Prognostic Staging System of American Joint Committee on Cancer 8th Edition for Breast Cancer: Comparisons with the Conventional Anatomic Staging System
- Introduction of 7th AJCC Cancer Staging: Gallbladder, Perihilar Bile Duct and Distal Common Bile Duct
- Comparison of the Differences in Survival Rates between the 7th and 8th Editions of the AJCC TNM Staging System for Gastric Adenocarcinoma: a Single-Institution Study of 5,507 Patients in Korea
- Evaluation of the American Joint Committee on Cancer (AJCC) 8th Edition Staging System for Hepatocellular Carcinoma in 1,008 Patients with Curative Resection
- Prognostic Validation of the American Joint Committee on Cancer 8th Staging System in 24,014 Korean Patients with Breast Cancer