J Korean Med Sci.
2020 Apr;35(15):e130. 10.3346/jkms.2020.35.e130.
Therapeutic Effects of Mesenchymal Stem Cells on a Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis Model
- Affiliations
-
- 1Department of Allergy and Clinical Immunology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Division of Pulmonary, Allergy and Critical Care Medicine, Konkuk University Medical Center, Seoul, Korea
- 3Department of Internal Medicine, Division of Allergy and Clinical Immunology, Armed Forces Capital Hospital, Seongnam, Korea
- 4Department of Internal Medicine, Division of Pulmonary, Inje University Haeundae Paik Hospital, Busan, Korea
- 5Department of Pulmonary, Allergy and Critical Care Medicine, Seongnam Citizens Medical Center, Seongnam, Korea
- KMID: 2500193
- DOI: http://doi.org/10.3346/jkms.2020.35.e130
Abstract
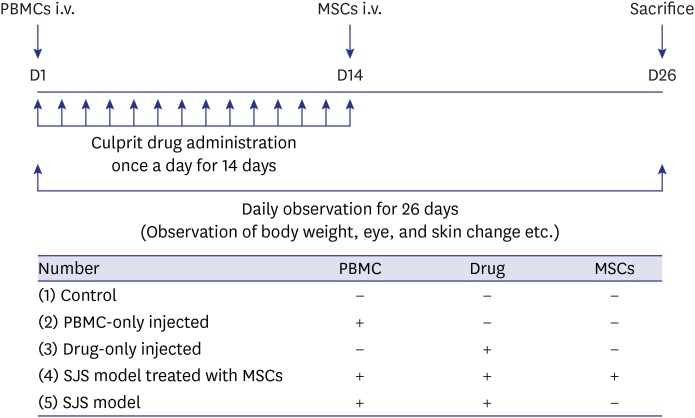
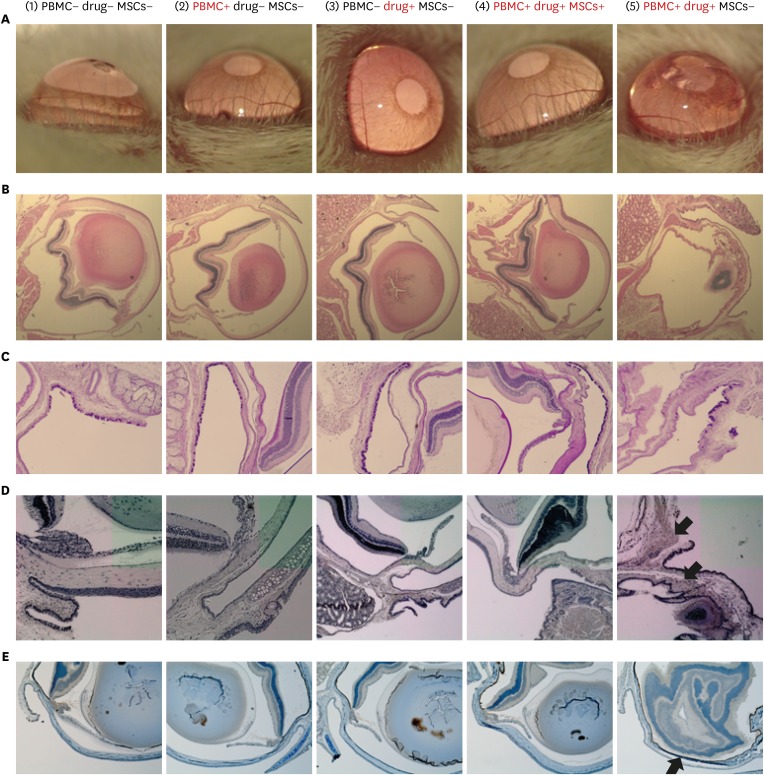
- Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) are the most severe cutaneous drug hypersensitivity reactions, which are unpredictable adverse drug reactions. SJS/TEN is associated with significant mortality and morbidity; however, effective treatment is difficult. Mesenchymal stem cells (MSCs) are well-known for their anti-inflammatory and tissue regeneration properties. The purpose of the present study was to verify whether MSCs could be applied for the treatment of SJS/TEN. We developed an SJS/TEN mouse model using peripheral blood mononuclear cells from a lamotrigine-induced SJS patient. MSCs were injected into the model to verify the treatment effect. In SJS model mice treated with MSCs, ocular damage rarely occurred, and apoptosis rate was significantly lower. We demonstrated a therapeutic effect of MSCs on SJS/TEN, with these cells presenting a potential novel therapy for the management of this disorder.
Keyword
Figure
Reference
-
1. Chen CB, Abe R, Pan RY, Wang CW, Hung SI, Tsai YG, et al. An updated review of the molecular mechanisms in drug hypersensitivity. J Immunol Res. 2018; 2018:6431694. PMID: 29651444.
Article2. Bellón T, Blanca M. The innate immune system in delayed cutaneous allergic reactions to medications. Curr Opin Allergy Clin Immunol. 2011; 11(4):292–298. PMID: 21659858.
Article3. Ueta M. Genetic predisposition to Stevens-Johnson syndrome with severe ocular surface complications. Cornea. 2015; 34(Suppl 11):S158–S165. PMID: 26448174.
Article4. White KD, Abe R, Ardern-Jones M, Beachkofsky T, Bouchard C, Carleton B, et al. SJS/TEN 2017: Building multidisciplinary networks to drive science and translation. J Allergy Clin Immunol Pract. 2018; 6(1):38–69. PMID: 29310768.
Article5. Egunsola O, Star K, Juhlin K, Kardaun SH, Choonara I, Sammons HM. Retrospective review of paediatric case reports of Stevens-Johnson syndrome and toxic epidermal necrolysis with lamotrigine from an international pharmacovigilance database. BMJ Paediatr Open. 2017; 1(1):e000039.
Article6. White KD, Chung WH, Hung SI, Mallal S, Phillips EJ. Evolving models of the immunopathogenesis of T cell-mediated drug allergy: the role of host, pathogens, and drug response. J Allergy Clin Immunol. 2015; 136(2):219–234. PMID: 26254049.7. Michels AW, Ostrov DA. New approaches for predicting T cell-mediated drug reactions: a role for inducible and potentially preventable autoimmunity. J Allergy Clin Immunol. 2015; 136(2):252–257. PMID: 26254052.8. Saito N, Yoshioka N, Abe R, Qiao H, Fujita Y, Hoshina D, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis mouse model generated by using PBMCs and the skin of patients. J Allergy Clin Immunol. 2013; 131(2):434–441.e1. PMID: 23111236.
Article9. Duscher D, Barrera J, Wong VW, Maan ZN, Whittam AJ, Januszyk M, et al. Stem cells in wound healing: the future of regenerative medicine? A mini-review. Gerontology. 2016; 62(2):216–225. PMID: 26045256.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- T Cell Mediated Drug Hypersensitivity: Stevens Johnson Syndrome and Toxic Epidermal Necrolysis
- A Case of Toxic Epidermal Necrolysis
- A comparative clinical study of toxic epidermal necrolysis and Stevens-Johnson syndrome
- Two Cases of HLA-B59(+) Stevens-Johnson Syndrome (SJS)-Toxic Epidermal Necrolysis (TEN) Associated with Methazolamide Treatment
- Toxic Epidermal Necrolysis and Stevens-Johnson Syndrome Caused by Topical Ophthalmic Use of Dorzolamide