World J Mens Health.
2020 Apr;38(2):151-163. 10.5534/wjmh.190044.
Bone Health Issues in Patients with Prostate Cancer: An Evidence-Based Review
- Affiliations
-
- 1Department of Urology, Eberhard Karls University, Tuebingen, Germany. Tilman.todenhoefer@med.uni-tuebingen.de
- KMID: 2471822
- DOI: http://doi.org/10.5534/wjmh.190044
Abstract
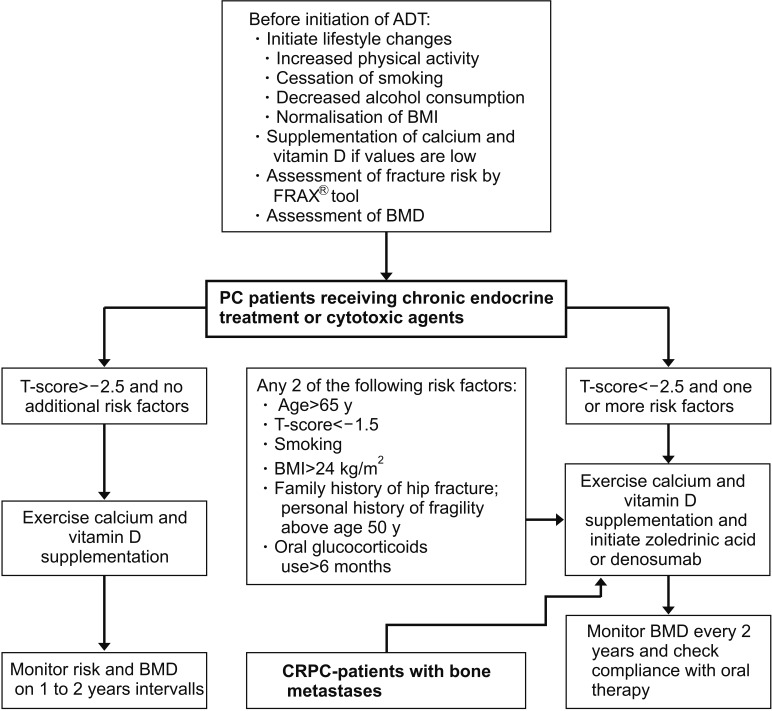
- Bone health in prostate cancer patients represents a prerequisite for acceptable quality of life and optimal outcome of this disease. The major threat for bone health in prostate cancer displays cancer treatment induced bone loss as well as the development of bone metastases. In recent years, several new pharmaceuticals targeting bone metabolism such as denosumab or androgen pathway targeting drugs (abiraterone acetate and enzalutamide) have been approved for the treatment of progressive disease aiming to interrupt the vicious circle of bone metastasis and aberrant bone resorption. This development raised the awareness of the pivotal role of bone health in prostate cancer and introduced (symptomatic) skeletal related events as an important end point in recent clinical trials. Bone targeted drugs have become standard of care in patients with metastatic castration resistant prostate cancer, their role in metastatic hormone sensitive prostate cancer has been discussed controversely. In oligometastatic prostate cancer patients several promising approaches in metastasis directed therapy, including conventional surgery, stereotactic ablative radiation and image-guided single-fraction robotic stereotactic radiosurgery (CyberKnife®) were launched but are not in routine clinical use until now caused by sparse clinical evidence.
Keyword
MeSH Terms
Figure
Reference
-
1. Gandaglia G, Karakiewicz PI, Briganti A, Passoni NM, Schiffmann J, Trudeau V, et al. Impact of the site of metastases on survival in patients with metastatic prostate cancer. Eur Urol. 2015; 68:325–334. PMID: 25108577.
Article2. Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000; 31:578–583. PMID: 10836297.
Article3. Ibrahim A, Scher N, Williams G, Sridhara R, Li N, Chen G, et al. Approval summary for zoledronic acid for treatment of multiple myeloma and cancer bone metastases. Clin Cancer Res. 2003; 9:2394–2399. PMID: 12855610.4. Howard LE, De Hoedt AM, Aronson WJ, Kane CJ, Amling CL, Cooperberg MR, et al. Do skeletal-related events predict overall survival in men with metastatic castration-resistant prostate cancer? Prostate Cancer Prostatic Dis. 2016; 19:380–384. PMID: 27377207.
Article5. Krupski TL, Foley KA, Baser O, Long S, Macarios D, Litwin MS. Health care cost associated with prostate cancer, androgen deprivation therapy and bone complications. J Urol. 2007; 178:1423–1428. PMID: 17706711.
Article6. Weinfurt KP, Li Y, Castel LD, Saad F, Timbie JW, Glendenning GA, et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol. 2005; 16:579–584. PMID: 15734776.
Article7. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014; 65:467–479. PMID: 24321502.
Article8. Taxel P, Faircloth E, Idrees S, Van Poznak C. Cancer treatment-induced bone loss in women with breast cancer and men with prostate cancer. J Endocr Soc. 2018; 2:574–588. PMID: 29942922.
Article9. de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010; 376:1147–1154. PMID: 20888992.
Article10. Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004; 351:1502–1512. PMID: 15470213.
Article11. Quach JM, Askmyr M, Jovic T, Baker EK, Walsh NC, Harrison SJ, et al. Myelosuppressive therapies significantly increase pro-inflammatory cytokines and directly cause bone loss. J Bone Miner Res. 2015; 30:886–897. PMID: 25418357.
Article12. Wissing MD. Chemotherapy- and irradiation-induced bone loss in adults with solid tumors. Curr Osteoporos Rep. 2015; 13:140–145. PMID: 25712619.
Article13. Msaouel P, Diamanti E, Tzanela M, Koutsilieris M. Luteinising hormone-releasing hormone antagonists in prostate cancer therapy. Expert Opin Emerg Drugs. 2007; 12:285–299. PMID: 17604502.
Article14. Hofbauer LC, Rachner TD, Coleman RE, Jakob F. Endocrine aspects of bone metastases. Lancet Diabetes Endocrinol. 2014; 2:500–512. PMID: 24880565.
Article15. Todenhöfer T, Stenzl A, Hofbauer LC, Rachner TD. Targeting bone metabolism in patients with advanced prostate cancer: current options and controversies. Int J Endocrinol. 2015; 2015:838202. PMID: 25802521.
Article16. Grossmann M, Cheung AS, Zajac JD. Androgens and prostate cancer; pathogenesis and deprivation therapy. Best Pract Res Clin Endocrinol Metab. 2013; 27:603–616. PMID: 24054933.
Article17. Drake MT, Khosla S. Male osteoporosis. Endocrinol Metab Clin North Am. 2012; 41:629–641. PMID: 22877433.
Article18. Briot K, Roux C. Glucocorticoid-induced osteoporosis. RMD Open. 2015; 1:e000014. PMID: 26509049.
Article19. Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007; 18:1319–1328. PMID: 17566815.
Article20. Cheung AS, Zajac JD, Grossmann M. Muscle and bone effects of androgen deprivation therapy: current and emerging therapies. Endocr Relat Cancer. 2014; 21:R371–R394. PMID: 25056176.
Article21. Saad F, Adachi JD, Brown JP, Canning LA, Gelmon KA, Josse RG, et al. Cancer treatment-induced bone loss in breast and prostate cancer. J Clin Oncol. 2008; 26:5465–5476. PMID: 18955443.
Article22. Eriksen EF. Treatment of osteopenia. Rev Endocr Metab Disord. 2012; 13:209–223. PMID: 21710179.
Article23. Wilson KM, Shui IM, Mucci LA, Giovannucci E. Calcium and phosphorus intake and prostate cancer risk: a 24-y follow-up study. Am J Clin Nutr. 2015; 101:173–183. PMID: 25527761.
Article24. Smith MR, Egerdie B, Hernández Toriz N, Feldman R, Tammela TL, Saad F, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009; 361:745–755. PMID: 19671656.
Article25. Serpa Neto A, Tobias-Machado M, Esteves MA, Senra MD, Wroclawski ML, Fonseca FL, et al. Bisphosphonate therapy in patients under androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2012; 15:36–44. PMID: 21894175.
Article26. Smith MR, Morton RA, Barnette KG, Sieber PR, Malkowicz SB, Rodriguez D, et al. Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2010; 184:1316–1321. PMID: 20723926.
Article27. Rachner TD, Göbel A, Benad-Mehner P, Hofbauer LC, Rauner M. Dickkopf-1 as a mediator and novel target in malignant bone disease. Cancer Lett. 2014; 346:172–177. PMID: 24462802.
Article28. García-Fontana B, Morales-Santana S, Varsavsky M, García-Martín A, García-Salcedo JA, Reyes-García R, et al. Sclerostin serum levels in prostate cancer patients and their relationship with sex steroids. Osteoporos Int. 2014; 25:645–651. PMID: 23903956.
Article29. Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016; 375:1532–1543. PMID: 27641143.
Article30. Lewiecki EM, Blicharski T, Goemaere S, Lippuner K, Meisner PD, Miller PD, et al. A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab. 2018; 103:3183–3193. PMID: 29931216.
Article31. Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003; 1653:1–24. PMID: 12781368.
Article32. Gomes RR Jr, Buttke P, Paul EM, Sikes RA. Osteosclerotic prostate cancer metastasis to murine bone are enhanced with increased bone formation. Clin Exp Metastasis. 2009; 26:641–651. PMID: 19421879.
Article33. Fizazi K, Massard C, Smith M, Rader M, Brown J, Milecki P, et al. Bone-related parameters are the main prognostic factors for overall survival in men with bone metastases from castration-resistant prostate cancer. Eur Urol. 2015; 68:42–50. PMID: 25449207.
Article34. Hahn NM, Yiannoutsos CT, Kirkpatrick K, Sharma J, Sweeney CJ. Failure to suppress markers of bone turnover on first-line hormone therapy for metastatic prostate cancer is associated with shorter time to skeletal-related event. Clin Genitourin Cancer. 2014; 12:33–40.e4. PMID: 24126237.
Article35. Oster G, Lamerato L, Glass AG, Richert-Boe KE, Lopez A, Chung K, et al. Natural history of skeletal-related events in patients with breast, lung, or prostate cancer and metastases to bone: a 15-year study in two large US health systems. Support Care Cancer. 2013; 21:3279–3286. PMID: 23884473.
Article36. Berish RB, Ali AN, Telmer PG, Ronald JA, Leong HS. Translational models of prostate cancer bone metastasis. Nat Rev Urol. 2018; 15:403–421. PMID: 29769644.
Article37. Nagle RB, Cress AE. Metastasis update: human prostate carcinoma invasion via tubulogenesis. Prostate Cancer. 2011; 2011:249290. PMID: 21949592.
Article38. Smith BN, Odero-Marah VA. The role of snail in prostate cancer. Cell Adh Migr. 2012; 6:433–441. PMID: 23076049.
Article39. Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002; 62:1832–1837. PMID: 11912162.40. Engl T, Relja B, Marian D, Blumenberg C, Müller I, Beecken WD, et al. CXCR4 chemokine receptor mediates prostate tumor cell adhesion through alpha5 and beta3 integrins. Neoplasia. 2006; 8:290–301. PMID: 16756721.41. McCabe NP, De S, Vasanji A, Brainard J, Byzova TV. Prostate cancer specific integrin alphavbeta3 modulates bone metastatic growth and tissue remodeling. Oncogene. 2007; 26:6238–6243. PMID: 17369840.42. Hall CL, Daignault SD, Shah RB, Pienta KJ, Keller ET. Dickkopf-1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasis. Prostate. 2008; 68:1396–1404. PMID: 18561248.
Article43. Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004; 96:879–882. PMID: 15173273.
Article44. Prentice A. Diet, nutrition and the prevention of osteoporosis. Public Health Nutr. 2004; 7:227–243. PMID: 14972062.
Article45. Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002; 94:1458–1468. PMID: 12359855.
Article46. Hegemann M, Maas M, Rausch S, Walz S, Bedke J, Stenzl A, et al. Current concepts and trends in the treatment of bone metastases in patients with advanced prostate cancer. Asian J Androl. 2019; 21:12–18.
Article47. James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. STAMPEDE investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016; 387:1163–1177. PMID: 26719232.48. Smith MR, Halabi S, Ryan CJ, Hussain A, Vogelzang N, Stadler W, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol. 2014; 32:1143–1150. PMID: 24590644.
Article49. Wirth M, Tammela T, Cicalese V, Gomez Veiga F, Delaere K, Miller K, et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur Urol. 2015; 67:482–491. PMID: 24630685.
Article50. Serefoglu EC, Tandogdu Z. Efficacy and safety of zoledronic acid in the treatment of glucocorticoid-induced osteoporosis. Ther Clin Risk Manag. 2010; 6:219–223. PMID: 20526439.
Article51. Himelstein AL, Foster JC, Khatcheressian JL, Roberts JD, Seisler DK, Novotny PJ, et al. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017; 317:48–58. PMID: 28030702.52. Cirak Y, Varol U, Atmaca H, Kisim A, Sezgin C, Karabulut B, et al. Zoledronic acid in combination with serine/threonine phosphatase inhibitors induces enhanced cytotoxicity and apoptosis in hormone-refractory prostate cancer cell lines by decreasing the activities of PP1 and PP2A. BJU Int. 2012; 110:E1147–E1154. PMID: 22882676.
Article53. Brubaker KD, Brown LG, Vessella RL, Corey E. Administration of zoledronic acid enhances the effects of docetaxel on growth of prostate cancer in the bone environment. BMC Cancer. 2006; 6:15. PMID: 16417633.
Article54. Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011; 377:813–822. PMID: 21353695.
Article55. Smith MR, Coleman RE, Klotz L, Pittman K, Milecki P, Ng S, et al. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann Oncol. 2015; 26:368–374. PMID: 25425475.
Article56. Patrick DL, Cleeland CS, von Moos R, Fallowfield L, Wei R, Öhrling K, et al. Pain outcomes in patients with bone metastases from advanced cancer: assessment and management with bone-targeting agents. Support Care Cancer. 2015; 23:1157–1168. PMID: 25533578.
Article57. Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012; 379:39–46. PMID: 22093187.
Article58. Body JJ, von Moos R, Niepel D, Tombal B. Hypocalcaemia in patients with prostate cancer treated with a bisphosphonate or denosumab: prevention supports treatment completion. BMC Urol. 2018; 18:81. PMID: 30236112.
Article59. Palafox M, Ferrer I, Pellegrini P, Vila S, Hernandez-Ortega S, Urruticoechea A, et al. RANK induces epithelial-mesenchymal transition and stemness in human mammary epithelial cells and promotes tumorigenesis and metastasis. Cancer Res. 2012; 72:2879–2888. PMID: 22496457.
Article60. Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013; 369:213–223. PMID: 23863050.
Article61. Walz S, Stenzl A, Todenhöfer T. Management skelettaler und nicht-skelettaler komplikationen beim prostatakarzinom. J Onkol. 2018; 11/2018:22–28.62. Coleman R, Fossa SD, Chodacki A, Wedel S, Bruland O, Staudacher K, et al. Time to first skeletal-related event (SRE) with radium-223 dichloride (Ra-223) in patients with castration-resistant prostate cancer (CRPC) and bone metastases: ALSYMPCA trial stratification factors analysis. Paper presented at: European Cancer Congress. 2013 Sep 27-Oct 1; Amsterdam, Netherlands. 2013. abstract #2876.63. Morris MJ, Loriot Y, Fizazi K, Sweeney C, Ryan CJ, Shevrin DH, et al. Effects of radium-223 (Ra-223) with docetaxel versus docetaxel alone on bone biomarkers in patients with bone-metastatic castration-resistant prostate cancer (CRPC): a phase I/IIa clinical trial. J Clin Oncol. 2017; 6(suppl):154.
Article64. Gourd E. EMA guidance on radium-223 dichloride in prostate cancer. Lancet Oncol. 2018; 19:e190. PMID: 29551361.
Article65. Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008; 26:242–245. PMID: 18182665.
Article66. Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004; 351:1513–1520. PMID: 15470214.
Article67. de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011; 364:1995–2005. PMID: 21612468.
Article68. Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, et al. COU-AA-301 Investigators. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012; 13:983–992. PMID: 22995653.
Article69. Logothetis CJ, Basch E, Molina A, Fizazi K, North SA, Chi KN, et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol. 2012; 13:1210–1217. PMID: 23142059.
Article70. Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013; 368:138–148. PMID: 23228172.
Article71. Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015; 16:152–160. PMID: 25601341.
Article72. Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017; 377:352–360. PMID: 28578607.
Article73. James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017; 377:338–351. PMID: 28578639.74. Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012; 367:1187–1197. PMID: 22894553.
Article75. Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014; 371:424–433. PMID: 24881730.
Article76. Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018; 378:2465–2474. PMID: 29949494.
Article77. Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018; 378:1408–1418. PMID: 29420164.
Article78. Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019; 380:1235–1246. PMID: 30763142.
Article79. Lutz S, Chow E. A review of recently published radiotherapy treatment guidelines for bone metastases: contrasts or convergence? J Bone Oncol. 2012; 1:18–23. PMID: 26909250.
Article80. Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J. ESMO Guidelines Working Group. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2014; 25 Suppl 3:iii124–iii137. PMID: 24782453.
Article81. Weiss RJ, Forsberg JA, Wedin R. Surgery of skeletal metastases in 306 patients with prostate cancer. Acta Orthop. 2012; 83:74–79. PMID: 22206449.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Guidelines for Evaluating Treatment Response Based on Bone Scan for Metastatic Castration-Resistant Prostate Cancer: Prostate Cancer Clinical Trial Working Group 3 Recommendations
- Questioning the evidence behind the Saturation Model for testosterone replacement therapy in prostate cancer
- The Treatments for Low-Risk Prostate Cancer
- Oxidative Stress, Diet and Prostate Cancer
- Prostate Cancer in Young Man