J Breast Cancer.
2019 Dec;22(4):599-612. 10.4048/jbc.2019.22.e46.
Omission of Chemotherapy for the Treatment of Mucinous Breast Cancer: A Nationwide Study from the Korean Breast Cancer Society
- Affiliations
-
- 1Division of Breast Surgery, Department of Surgery, Hanyang University Guri Hospital, Hanyang University College of Medicine, Seoul, Korea.
- 2Division of Breast Surgery, Department of Surgery, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. wcpark@catholic.ac.kr
- 3Division of Breast Surgery, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Biostatistical Consulting and Research Lab, Medical Research Collaborating Center, Hanyang University, Seoul, Korea.
- KMID: 2470896
- DOI: http://doi.org/10.4048/jbc.2019.22.e46
Abstract
- PURPOSE
Mucinous breast carcinoma (MBC) is a rare type of breast cancer. Although patients with MBC may have a better prognosis than that of patients with invasive ductal carcinoma, many clinicians administer adjuvant chemotherapy regimens similar to those for other breast tumors. Using data from a nationwide clinical database, this study evaluated the significance of adjuvant systemic chemotherapy and whether it can be omitted in MBC patients.
METHODS
We included 3,076 patients with a diagnosis of MBC recorded in the Korean Breast Cancer Registry between January 1990 and August 2016. We used the Kaplan-Meier method to analyze breast cancer-specific survival (BCCS) and overall survival (OS). Multivariate analysis was performed using a Cox proportional hazard ratio (HR) model to estimate the adjusted HR for each prognostic factor.
RESULTS
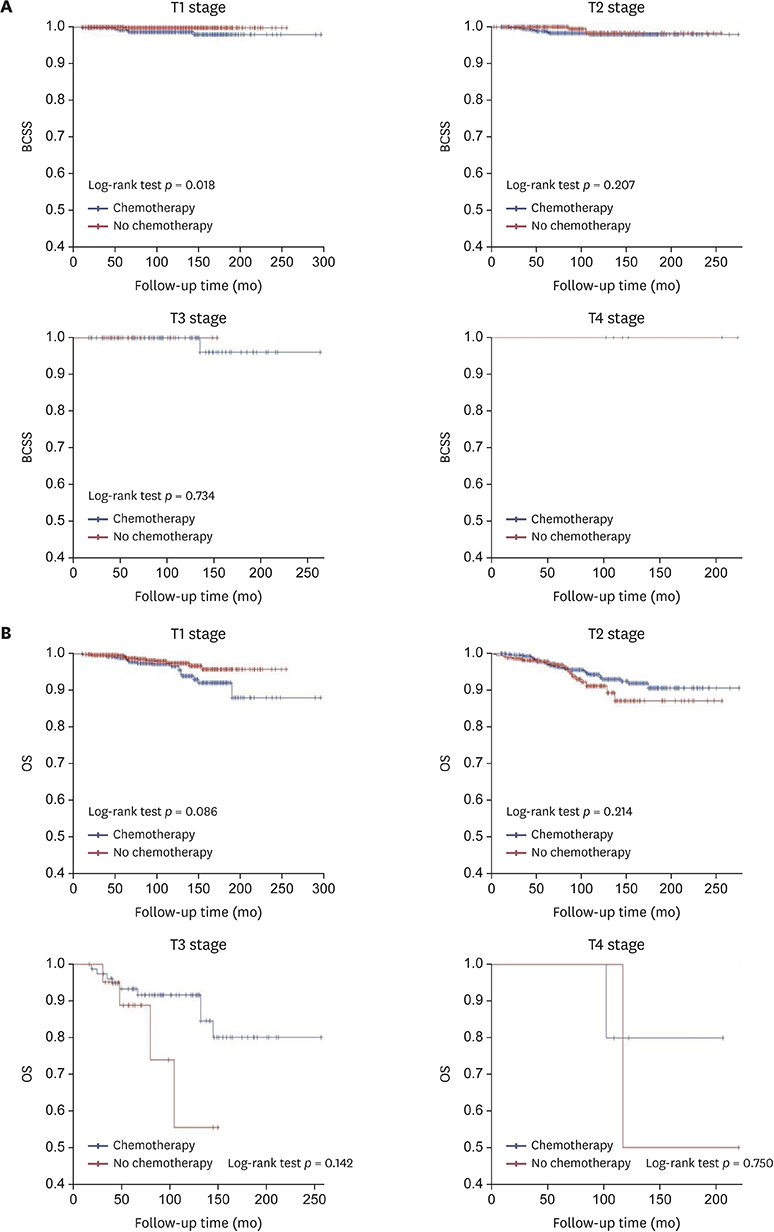
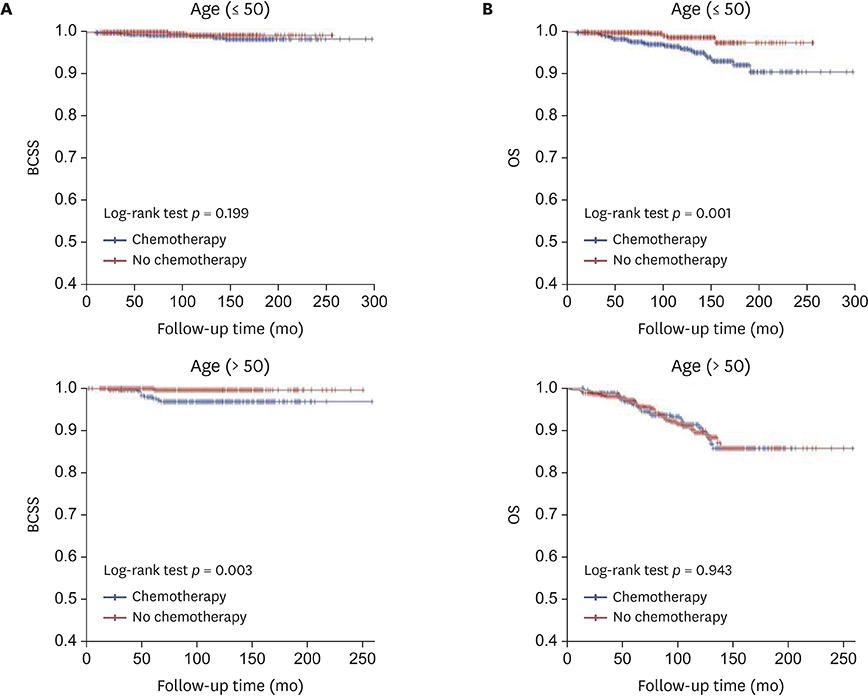
A total of 2,988 MBC patients were enrolled and followed-up for a median of 100 months (range, 2-324 months). Multivariate analysis revealed that axillary lymph node (ALN) metastasis and estrogen receptor (ER) negativity were significant prognostic factors for BCSS. Meanwhile, old age, pathologic tumor stage, and ALN metastasis were significant prognostic factors for OS. Subgroup analysis of ER-positive MBC showed that ALN metastasis was a significant prognostic factor for BCSS. Additionally, old age, pathologic tumor stage, and ALN metastasis were prognostic factors for OS. Ultimately, ALN metastasis was the most statistically significant prognostic factor for MBC. However, chemotherapy had no significant effect on BCSS and OS. The Kaplan-Meier curves of BCSS and OS based on pathologic tumor and nodal stages and age revealed that chemotherapy did not statistically significantly improve prognosis, except for the N3 stage.
CONCLUSION
Our large retrospective analysis revealed that adjuvant chemotherapy provided little benefit to improve the prognosis of most ER-positive MBC patients. Therefore, chemotherapy can be omitted in the treatment of most ER-positive MBC.
MeSH Terms
Figure
Reference
-
1. Azzopardi JG, Ahmed A, Millis RR. Problems in breast pathology. Major Probl Pathol. 1979; 11:i–xvi.2. Fentiman IS, Millis RR, Smith P, Ellul JP, Lampejo O. Mucoid breast carcinomas: histology and prognosis. Br J Cancer. 1997; 75:1061–1065.
Article3. Komaki K, Sakamoto G, Sugano H, Morimoto T, Monden Y. Mucinous carcinoma of the breast in Japan. A prognostic analysis based on morphologic features. Cancer. 1988; 61:989–996.
Article4. Gallager HS. Pathologic types of breast cancer: their prognoses. Cancer. 1984; 53:Suppl. 623–629.
Article5. Lacroix-Triki M, Suarez PH, MacKay A, Lambros MB, Natrajan R, Savage K, et al. Mucinous carcinoma of the breast is genomically distinct from invasive ductal carcinomas of no special type. J Pathol. 2010; 222:282–298.
Article6. Bae SY, Choi MY, Cho DH, Lee JE, Nam SJ, Yang JH. Mucinous carcinoma of the breast in comparison with invasive ductal carcinoma: clinicopathologic characteristics and prognosis. J Breast Cancer. 2011; 14:308–313.
Article7. Rasmussen BB, Rose C, Christensen IB. Prognostic factors in primary mucinous breast carcinoma. Am J Clin Pathol. 1987; 87:155–160.
Article8. Avisar E, Khan MA, Axelrod D, Oza K. Pure mucinous carcinoma of the breast: a clinicopathologic correlation study. Ann Surg Oncol. 1998; 5:447–451.
Article9. Di Saverio S, Gutierrez J, Avisar E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat. 2008; 111:541–547.
Article10. Goetz MP, Gradishar WJ, Anderson BO, Abraham J, Aft R, Allison KH, et al. NCCN guidelines insights: breast cancer, version 3.2018. J Natl Compr Canc Netw. 2019; 17:118–126.11. Fu J, Wu L, Jiang M, Li D, Jiang T, Hong Z, et al. Clinical Nomogram for predicting survival outcomes in early mucinous breast cancer. PLoS One. 2016; 11:e0164921.
Article12. Pan B, Yao R, Shi J, Xu QQ, Zhou YD, Mao F, et al. Prognosis of subtypes of the mucinous breast carcinoma in Chinese women: a population-based study of 32-year experience (1983–2014). Oncotarget. 2016; 7:38864–38875.
Article13. Zhang M, Teng XD, Guo XX, Zhao JS, Li ZG. Clinicopathological characteristics and prognosis of mucinous breast carcinoma. J Cancer Res Clin Oncol. 2014; 140:265–269.
Article14. Cao AY, He M, Liu ZB, Di GH, Wu J, Lu JS, et al. Outcome of pure mucinous breast carcinoma compared to infiltrating ductal carcinoma: a population-based study from China. Ann Surg Oncol. 2012; 19:3019–3027.
Article15. Park EH, Min SY, Kim Z, Yoon CS, Jung KW, Nam SJ, et al. Basic facts of breast cancer in Korea in 2014: the 10-year overall survival progress. J Breast Cancer. 2017; 20:1–11.
Article16. Kang SY, Kim YS, Kim Z, Kim HY, Lee SK, Jung KW, et al. Basic findings regarding breast cancer in Korea in 2015: data from a breast cancer registry. J Breast Cancer. 2018; 21:1–10.
Article17. Diab SG, Clark GM, Osborne CK, Libby A, Allred DC, Elledge RM. Tumor characteristics and clinical outcome of tubular and mucinous breast carcinomas. J Clin Oncol. 1999; 17:1442–1448.
Article18. Northridge ME, Rhoads GG, Wartenberg D, Koffman D. The importance of histologic type on breast cancer survival. J Clin Epidemiol. 1997; 50:283–290.
Article19. Silverberg SG, Kay S, Chitale AR, Levitt SH. Colloid carcinoma of the breast. Am J Clin Pathol. 1971; 55:355–363.
Article20. Azzopardi JG, Chepick OF, Hartmann WH, Jafarey NA, Llombart-Bosch A, Ozzello L, et al. The World Health Organization histological typing of breast tumors--second edition. Am J Clin Pathol. 1982; 78:806–816.
Article21. Skotnicki P, Sas-Korczynska B, Strzepek L, Jakubowicz J, Blecharz P, Reinfuss M, et al. Pure and mixed mucinous carcinoma of the breast: a comparison of clinical outcomes and treatment results. Breast J. 2016; 22:529–534.
Article22. Rasmussen BB. Human mucinous breast carcinomas and their lymph node metastases. A histological review of 247 cases. Pathol Res Pract. 1985; 180:377–382.23. Memis A, Ozdemir N, Parildar M, Ustun EE, Erhan Y. Mucinous (colloid) breast cancer: mammographic and US features with histologic correlation. Eur J Radiol. 2000; 35:39–43.
Article24. Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015; 373:2005–2014.
Article25. Piccart M, Rutgers E, van't Veer L, Sleets L, Delaloge S, Viale G, et al. Primary analysis of the EORTC 10041/ BIG 3-04 MINDACT study: a prospective, randomized study evaluating the clinical utility of the 70-gene signature (MammaPrint) combined with common clinical-pathological criteria for selection of patients for adjuvant chemotherapy in breast cancer with 0 to 3 positive nodes. Cancer Res. 2016; 76:Suppl 14. CT039.26. Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006; 24:3726–3734.
Article27. Nielsen TO, Jensen MB, Burugu S, Gao D, Jørgensen CL, Balslev E, et al. High-risk premenopausal luminal a breast cancer patients derive no benefit from adjuvant cyclophosphamide-based chemotherapy: results from the DBCG77B clinical trial. Clin Cancer Res. 2017; 23:946–953.
Article28. Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009; 360:790–800.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mono- and Combination Chemotherapy for Metastatic Breast Cancer: An Increamental Step Forward
- Breast Cancer During Pregnancy
- Surgery of Breast Cancer during the Last 5 Years: More Sophisticated and Specialized?
- The Highligts of 28th Annual Meeting of San Antonio Breast Cancer Symposium
- Breast Cancer during Pregnancy