J Lipid Atheroscler.
2020 Jan;9(1):172-183. 10.12997/jla.2020.9.1.172.
Genetic Architecture of Circulating Very-Long-Chain (C24:0 and C22:0) Ceramide Concentrations
- Affiliations
-
- 1Diabetic Cardiovascular Disease Center, Cardiovascular Division, Department of Medicine, Washington University School of Medicine, St. Louis, MO, USA. lpeterso@wustl.edu
- 2Department of Biostatistics and Epidemiology, Boston University School of Public Health, Boston, MA, USA.
- 3Division of Cardiovascular Medicine and Epidemiology, Vanderbilt University Medical Center, Nashville, TN, USA.
- 4Framingham Heart Study, Framingham, MA, USA.
- 5Section of Preventive Medicine and Epidemiology, Department of Medicine, Boston University, Boston, MA, USA.
- 6Section of Cardiology, Department of Medicine, Boston University, Boston, MA, USA.
- KMID: 2470809
- DOI: http://doi.org/10.12997/jla.2020.9.1.172
Abstract
OBJECTIVE
Total ceramide concentrations are linked with increased insulin resistance and cardiac dysfunction. However, recent studies have demonstrated that plasma concentrations of specific very-long-chain fatty ceramides (C24:0 and C22:0) are associated with a reduced incidence of coronary heart disease and all-cause mortality. We hypothesized that specific genetic loci are associated with plasma C22:0 and C24:0 concentrations.
METHODS
Heritability and genome-wide association studies of plasma C24:0 and C22:0 ceramide concentrations were performed among 2,217 participants in the Framingham Heart Study Offspring Cohort, adjusting for cardiovascular risk factor covariates and cardiovascular drug treatment.
RESULTS
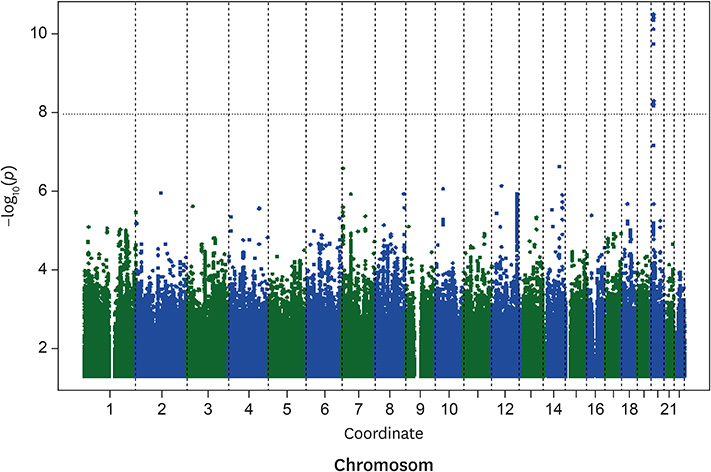
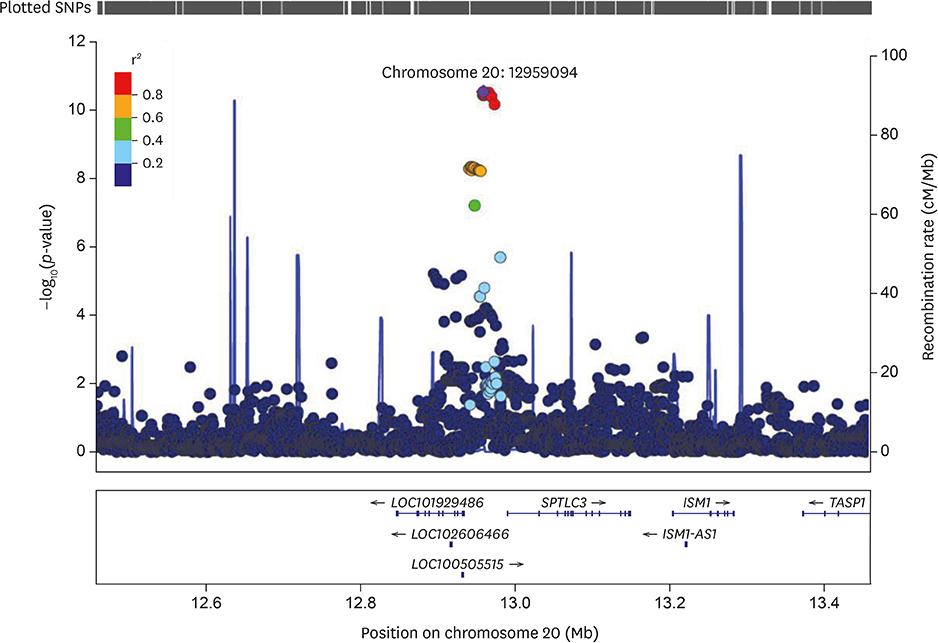
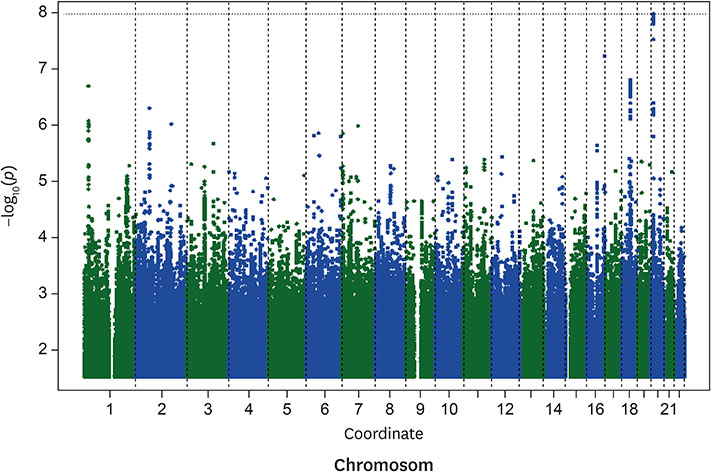
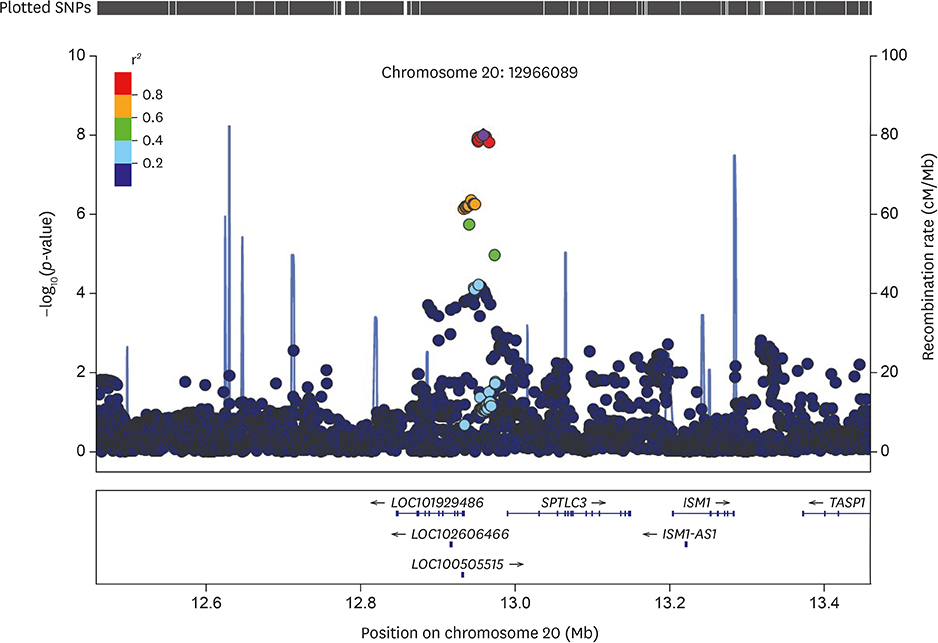
The multivariable-adjusted heritability for C22:0 and C24:0 ceramides was 0.42 (standard error [SE], 0.07; p=1.8E-9) and 0.25 (SE, 0.08; p=0.00025), respectively. Nineteen single nucleotide polymorphisms (SNPs), all on chromosome 20, significantly associated with C22:0 concentrations; the closest gene to these variants was SPTLC3. The lead SNP (rs4814175) significantly associated with 3% lower plasma C22:0 concentrations (p=2.83E-11). Nine SNPs, all on chromosome 20 and close to SPTLC3, were significantly associated with C24:0 ceramide concentrations. All 9 were also significantly related to plasma C22:0 levels. The lead SNP (rs168622) was significantly associated with 10% lower plasma C24:0 ceramide concentrations (p=9.94E-09).
CONCLUSION
SNPs near the SPTLC3 gene, which encodes serine palmitoyltransferase long chain base subunit 3 (SPTLC3; part of the enzyme that catalyzes the rate-limiting step of de novo sphingolipid synthesis) were associated with plasma C22:0 and C24:0 ceramide concentrations. These results are biologically plausible and suggest that SPTLC3 may be a potential therapeutic target for C24:0 and C22:0 ceramide modulation.
MeSH Terms
Figure
Reference
-
1. Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA. Lipid mediators of insulin resistance. Nutr Rev. 2007; 65:S39–S46.
Article2. Ussher JR, Koves TR, Cadete VJ, Zhang L, Jaswal JS, Swyrd SJ, et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes. 2010; 59:2453–2464.
Article3. Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, et al. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001; 107:813–822.
Article4. Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008; 49:2101–2112.
Article5. Chokshi A, Drosatos K, Cheema FH, Ji R, Khawaja T, Yu S, et al. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation. 2012; 125:2844–2853.
Article6. Peterson LR, Xanthakis V, Duncan MS, Gross S, Friedrich N, Völzke H, et al. Ceramide remodeling and risk of cardiovascular events and mortality. J Am Heart Assoc. 2018; 7:e007931.
Article7. Laaksonen R, Ekroos K, Sysi-Aho M, Hilvo M, Vihervaara T, Kauhanen D, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J. 2016; 37:1967–1976.
Article8. Tarasov K, Ekroos K, Suoniemi M, Kauhanen D, Sylvänne T, Hurme R, et al. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J Clin Endocrinol Metab. 2014; 99:E45–E52.9. Brice SE, Cowart LA. Sphingolipid Metabolism and analysis in metabolic disease. In : Cowart LA, editor. Sphingolipids and metabolic diseases. New York (NY): Landes Bioscience/Springer Science+Business Media, LLD;2011. p. 1–17.10. Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975; 4:518–525.
Article11. Jiang H, Hsu FF, Farmer MS, Peterson LR, Schaffer JE, Ory DS, et al. Development and validation of LC-MS/MS method for determination of very long acyl chain (C22:0 and C24:0) ceramides in human plasma. Anal Bioanal Chem. 2013; 405:7357–7365.
Article12. Chen MH, Yang Q. RVFam: an R package for rare variant association analysis with family data. Bioinformatics. 2016; 32:624–626.
Article13. de Mello VD, Lankinen M, Schwab U, Kolehmainen M, Lehto S, Seppänen-Laakso T, et al. Link between plasma ceramides, inflammation and insulin resistance: association with serum IL-6 concentration in patients with coronary heart disease. Diabetologia. 2009; 52:2612–2615.
Article14. Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000; 97:1784–1789.
Article15. Yu J, Pan W, Shi R, Yang T, Li Y, Yu G, et al. Ceramide is upregulated and associated with mortality in patients with chronic heart failure. Can J Cardiol. 2015; 31:357–363.
Article16. Russo SB, Tidhar R, Futerman AH, Cowart LA. Myristate-derived d16:0 sphingolipids constitute a cardiac sphingolipid pool with distinct synthetic routes and functional properties. J Biol Chem. 2013; 288:13397–13409.
Article17. Lopez X, Goldfine AB, Holland WL, Gordillo R, Scherer PE. Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. J Pediatr Endocrinol Metab. 2013; 26:995–998.
Article18. Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009; 58:337–343.
Article19. Illig T, Gieger C, Zhai G, Römisch-Margl W, Wang-Sattler R, Prehn C, et al. A genome-wide perspective of genetic variation in human metabolism. Nat Genet. 2010; 42:137–141.
Article20. Mirkov S, Myers JL, Ramírez J, Liu W. SNPs affecting serum metabolomic traits may regulate gene transcription and lipid accumulation in the liver. Metabolism. 2012; 61:1523–1527.
Article21. Ogretmen B. Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer. 2018; 18:33–50.
Article22. Morad SA, Cabot MC. Ceramide-orchestrated signalling in cancer cells. Nat Rev Cancer. 2013; 13:51–65.
Article23. Sassa T, Suto S, Okayasu Y, Kihara A. A shift in sphingolipid composition from C24 to C16 increases susceptibility to apoptosis in HeLa cells. Biochim Biophys Acta. 2012; 1821:1031–1037.
Article24. Park JW, Park WJ, Futerman AH. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim Biophys Acta. 2014; 1841:671–681.
Article25. Stiban J, Perera M. Very long chain ceramides interfere with C16-ceramide-induced channel formation: a plausible mechanism for regulating the initiation of intrinsic apoptosis. Biochim Biophys Acta. 2015; 1848:561–567.
Article26. Hicks AA, Pramstaller PP, Johansson A, Vitart V, Rudan I, Ugocsai P, et al. Genetic determinants of circulating sphingolipid concentrations in European populations. PLoS Genet. 2009; 5:e1000672.
Article27. Lemaitre RN, King IB, Kabagambe EK, Wu JH, McKnight B, Manichaikul A, et al. Genetic loci associated with circulating levels of very long-chain saturated fatty acids. J Lipid Res. 2015; 56:176–184.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reduced ceramides are associated with acute rejection in liver transplant patients and skin and hepatocyte transplant mice
- A Korean boy with atypical X-linked adrenoleukodystrophy confirmed by an unpublished mutation of ABCD1
- The Complex Tail of Circulating Sphingolipids in Atherosclerosis and Cardiovascular Disease
- Ceramide-Induced Apoptosis in Cultured Keratocyte
- The Apoptosis induced by Ceramide and Phytoceramide in the Lens Epithelial Cell