J Korean Soc Radiol.
2020 Jan;81(1):81-100. 10.3348/jksr.2020.81.1.81.
MR Neurography: Current Several Issues for Novice Radiologists
- Affiliations
-
- 1Department of Radiology, Dong-A University, Busan, Korea. hdhdoc@dau.ac.kr
- KMID: 2469184
- DOI: http://doi.org/10.3348/jksr.2020.81.1.81
Abstract
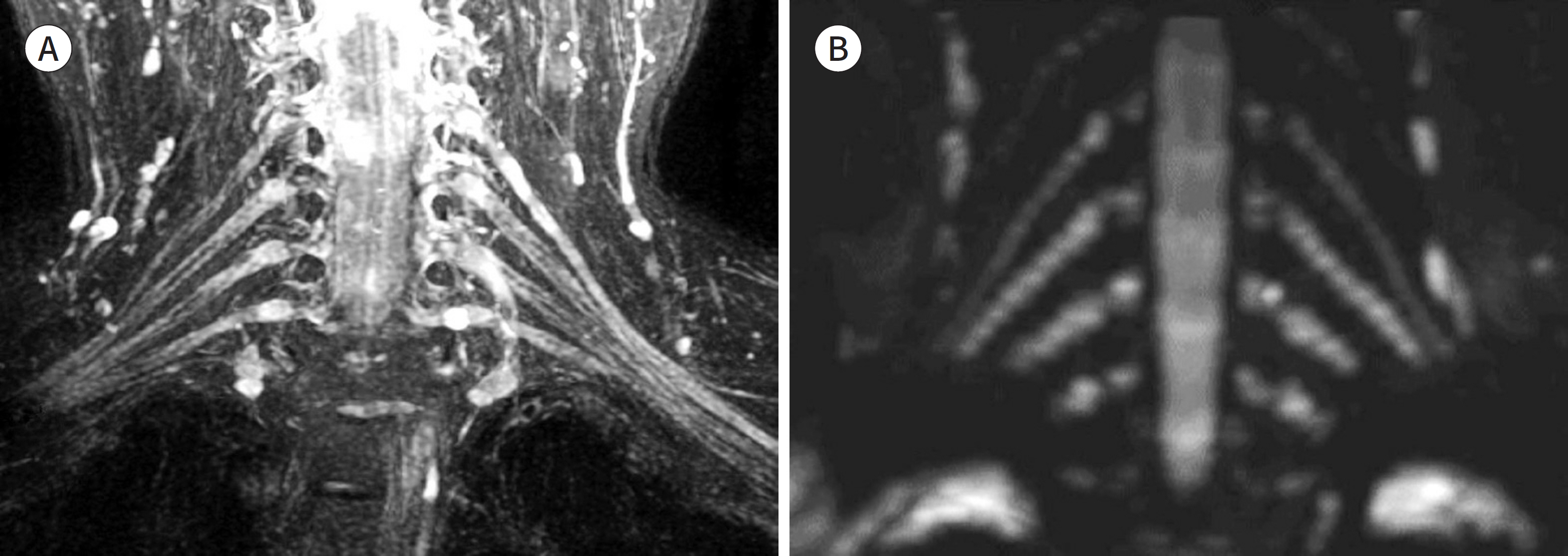
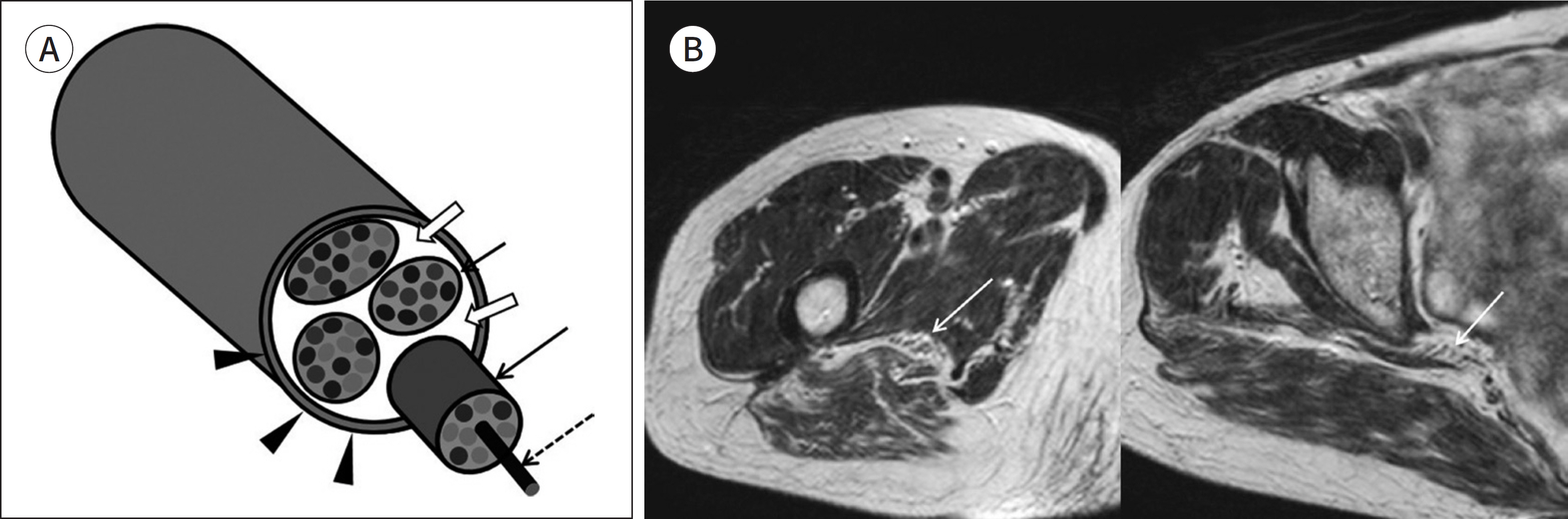
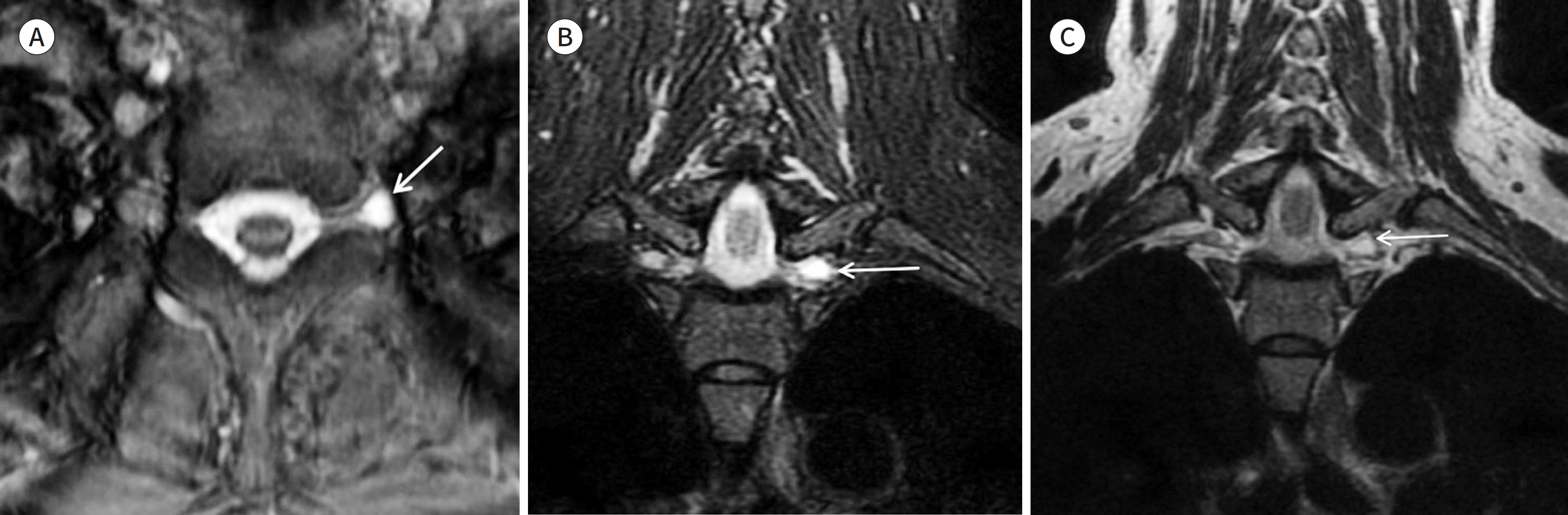
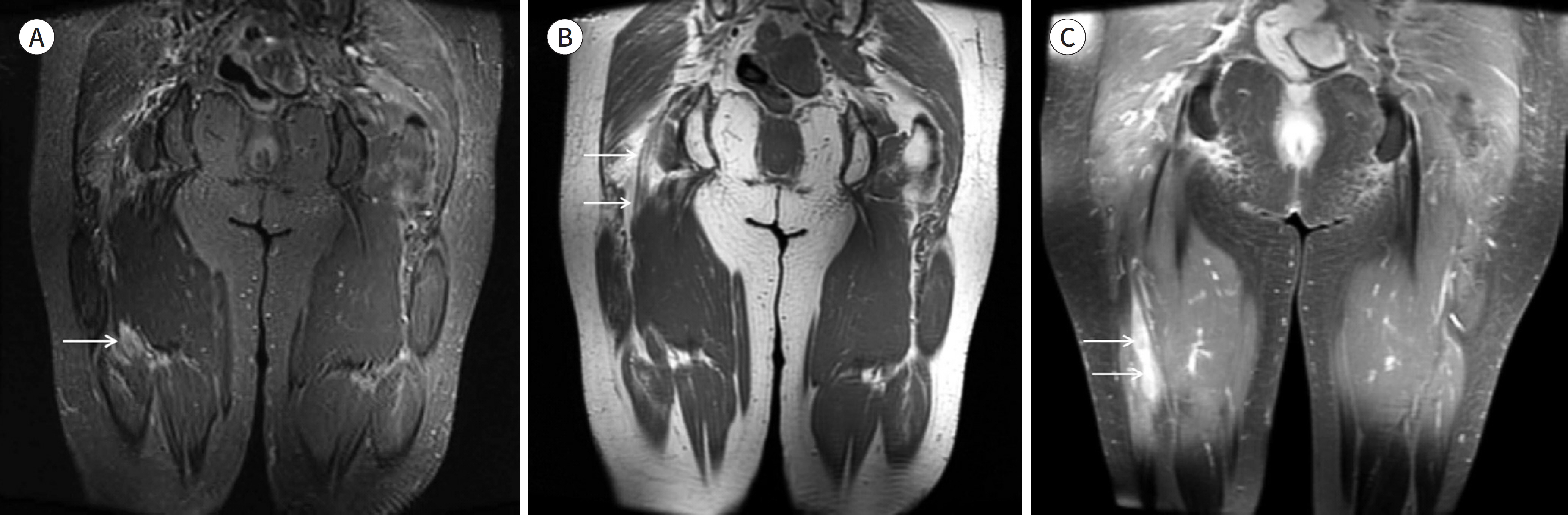
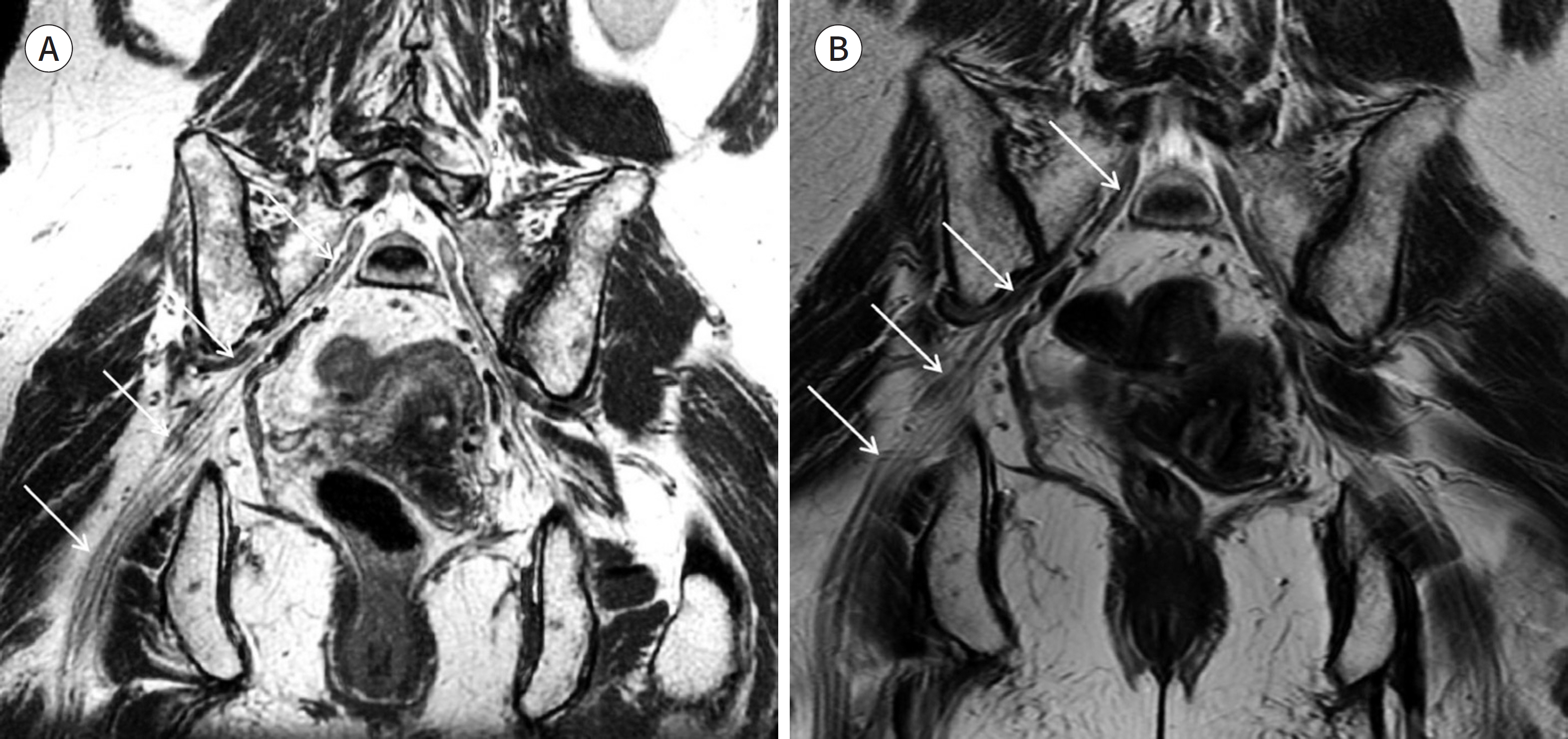
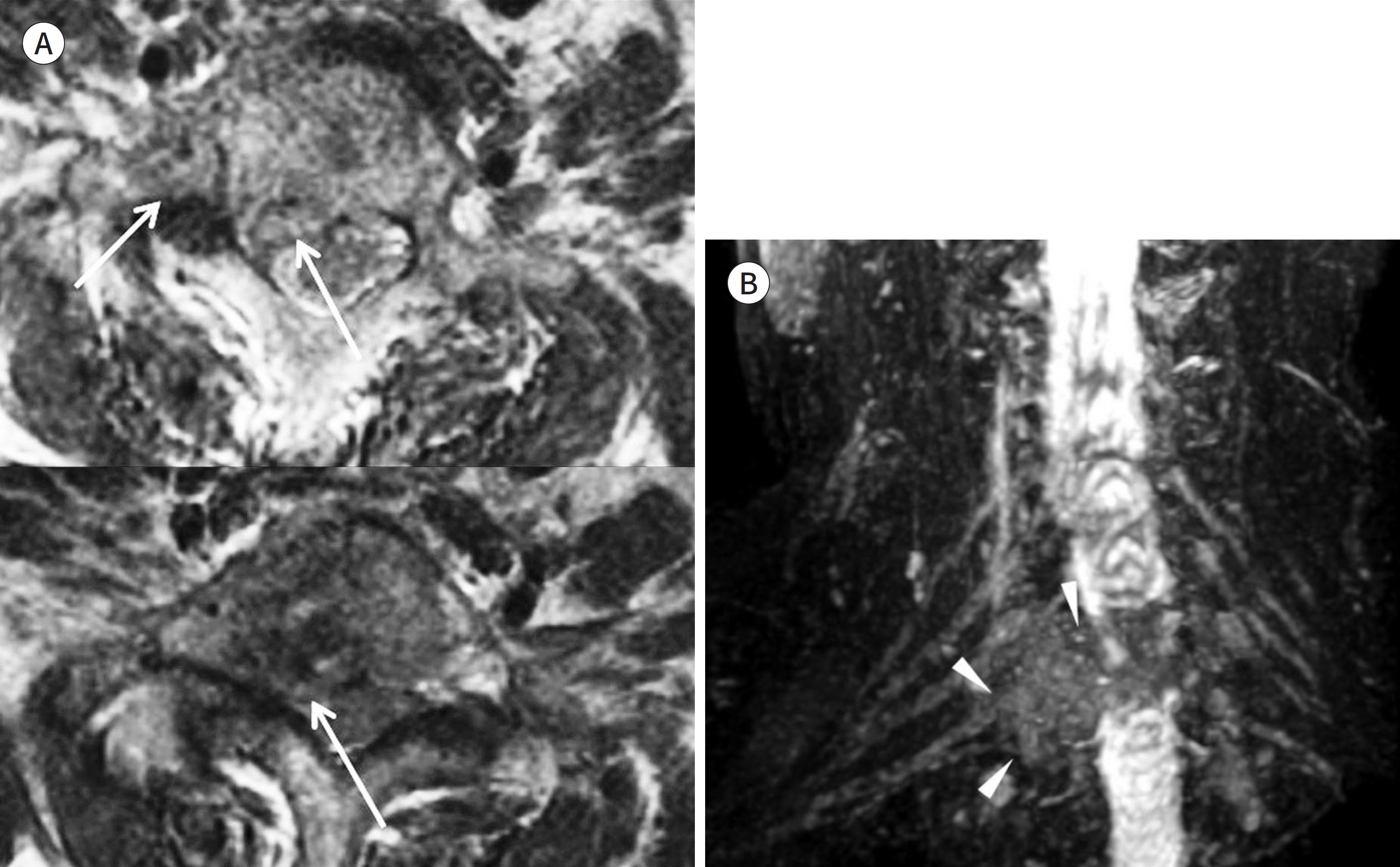
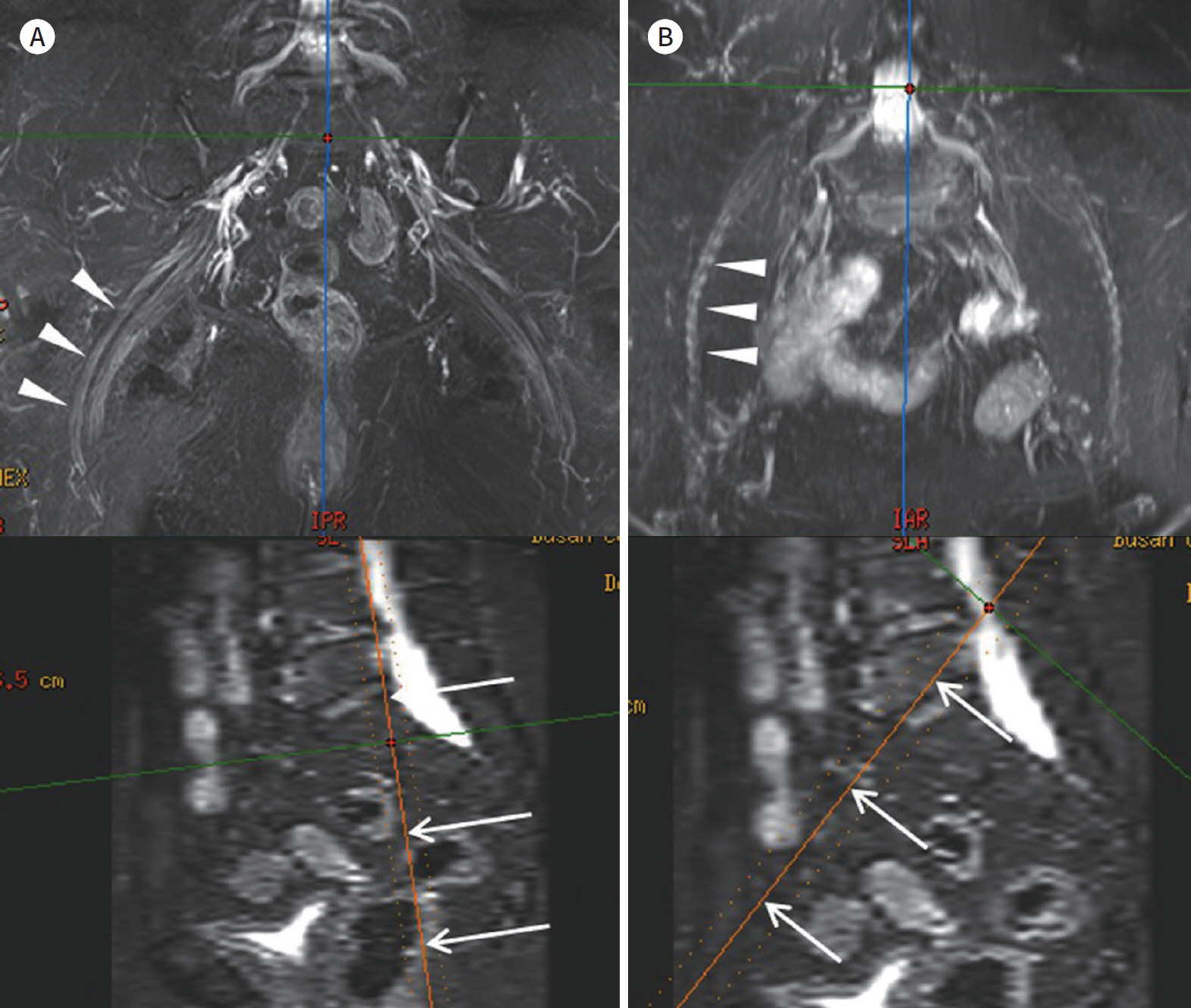
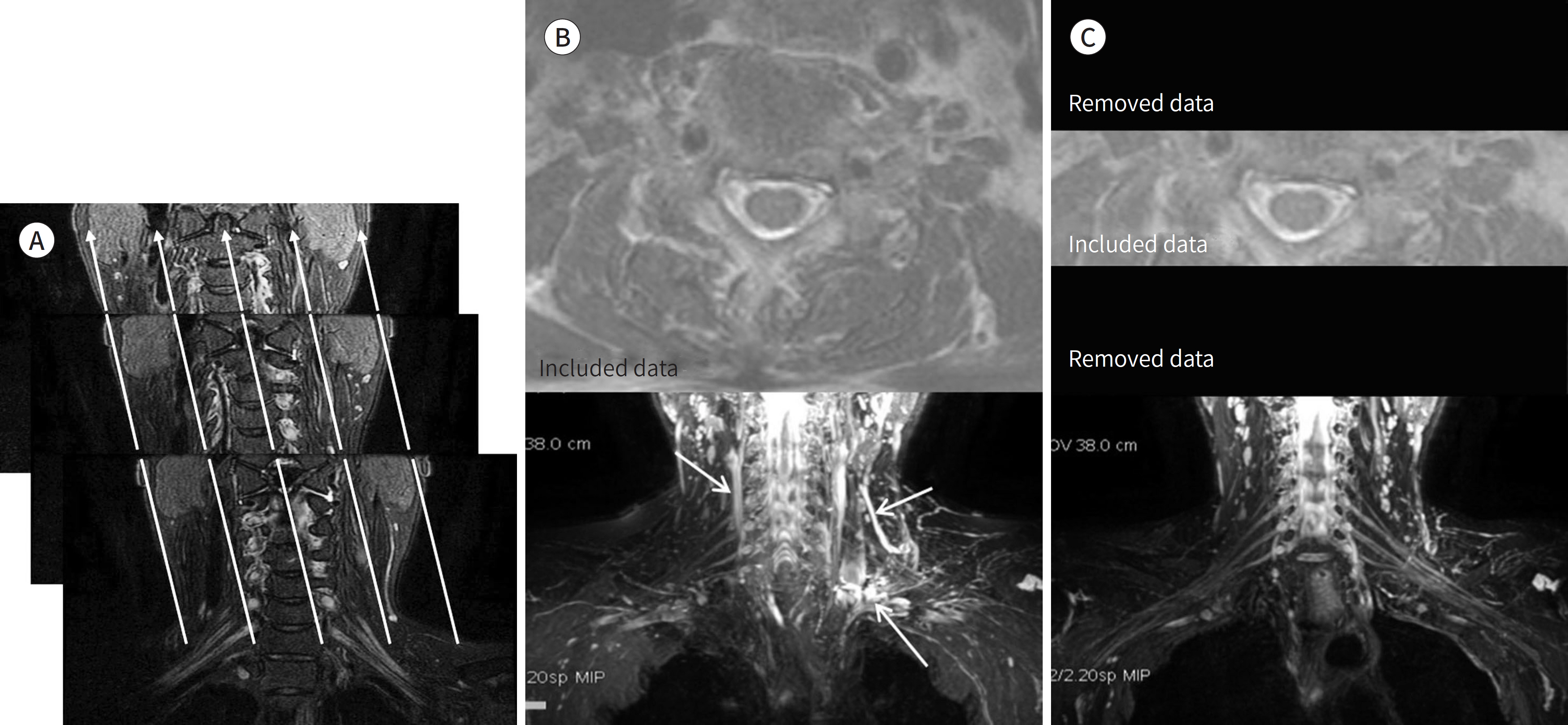
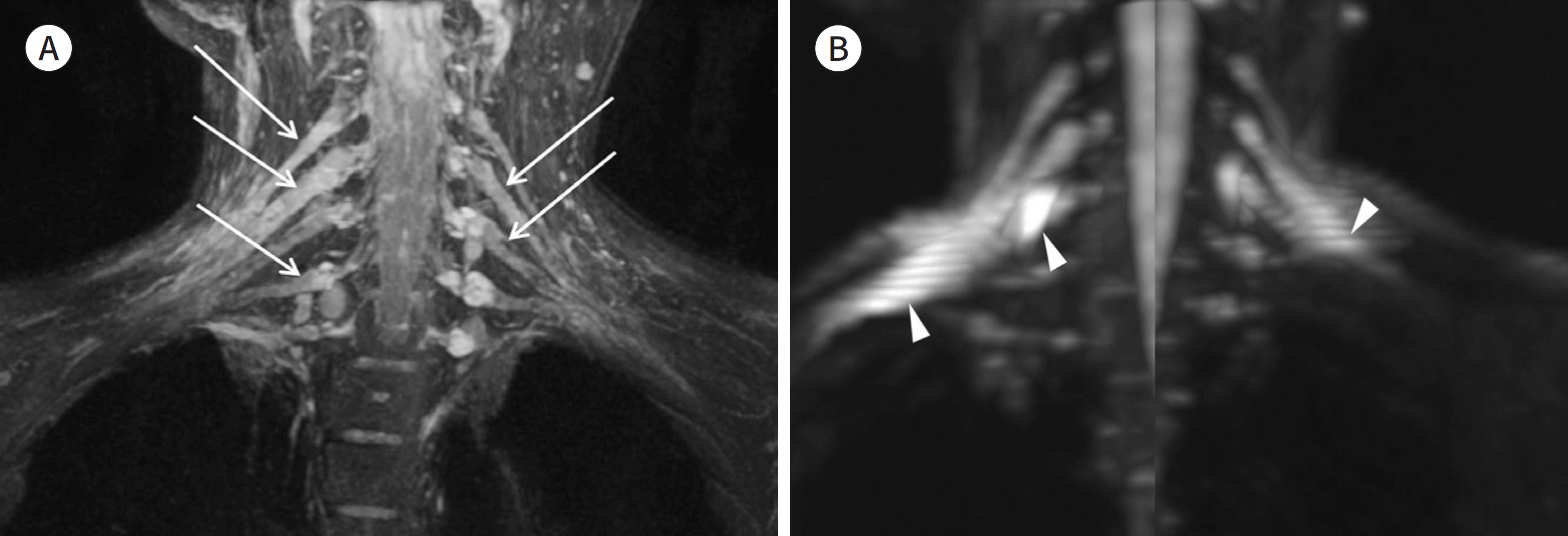
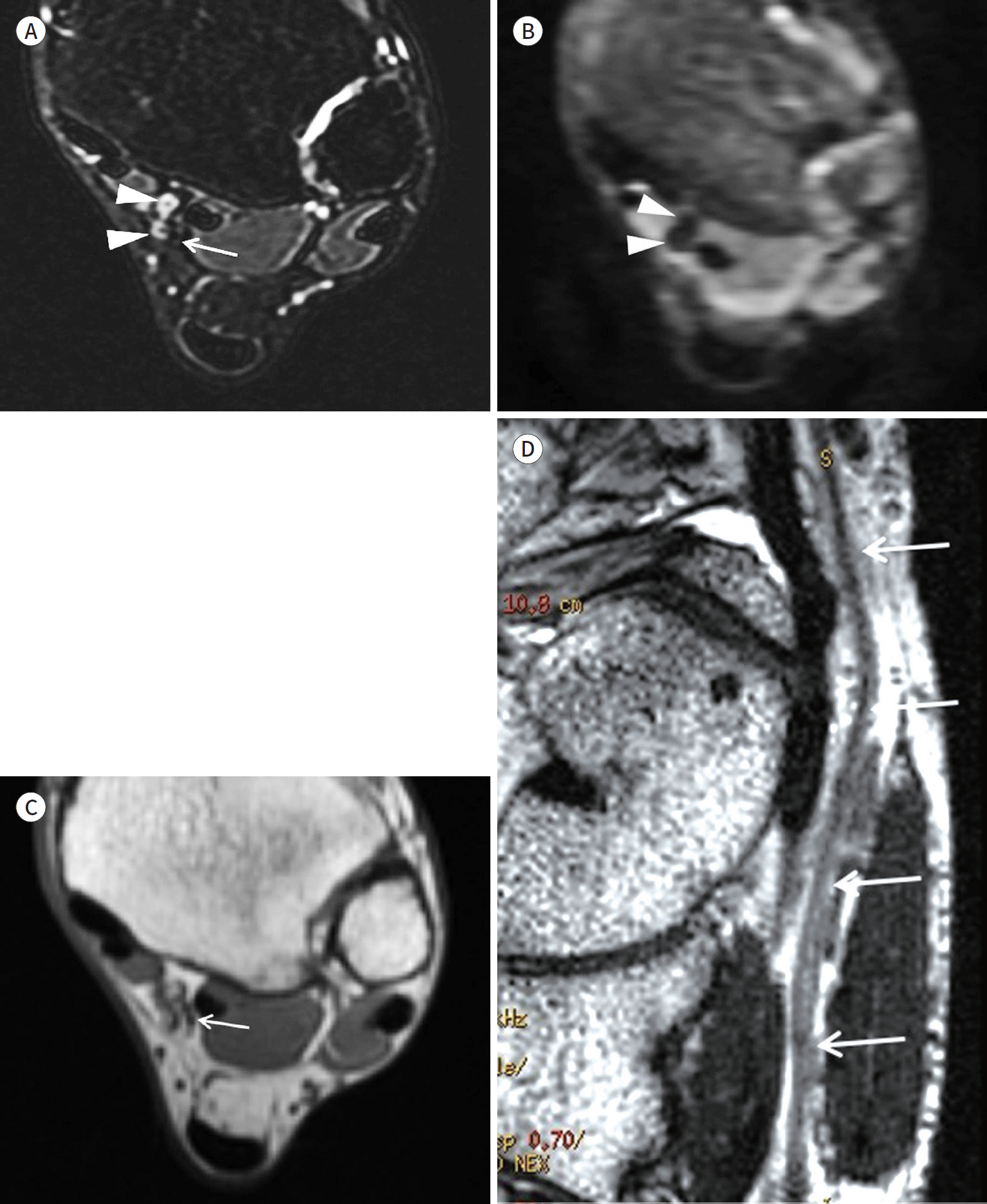
- Magnetic resonance neurography (MRN) has been increasingly used in recent years for the assessment of peripheral neuropathies. Fat suppression T2-weighted imaging (T2WI) and diffusion-weighted imaging (DWI) have typically been used to provide high contrast MRN. Isotropic 3-dimensional (3D) sequences with fast spin echo, post-processing imaging techniques, and fast imaging methods, among others, allow good visualization of peripheral nerves that have a small diameter, complex anatomy, and oblique course within a reasonable scan time. However, there are still several issues when performing high contrast and high resolution MRN including standard sequence; fat saturation techniques; balance between resolution, field of view, and slice thickness; post-processing techniques; 2D vs. 3D image acquisition; different T2 contrasts between proximal and distal nerves; high T2 signal intensity of adjacent veins or joint fluid; geometric distortion; and appropriate p-values on DWI. The proper understanding of these issues will help novice radiologists evaluate peripheral neuropathies using MRN.
MeSH Terms
Figure
Reference
-
1. Chhabra A, Lee PP, Bizzell C, Soldatos T. 3 Tesla MR neurography–technique, interpretation, and pitfalls. Skeletal Radiol. 2011; 40:1249–1260.
Article2. Ohana M, Moser T, Moussaouï A, Kremer S, Carlier RY, Liverneaux P, et al. Current and future imaging of the peripheral nervous system.Diagn Interv Imaging. 2014; 95:17–26.3. Donovan A, Rosenberg ZS, Cavalcanti CF. MR imaging of entrapment neuropathies of the lower extremity. Part 2. The knee, leg, ankle, and foot.Radiographics. 2010; 30:1001–1019.4. Andreisek G, Crook DW, Burg D, Marincek B, Weishaupt D. Peripheral neuropathies of the median, radial, and ulnar nerves: MR imaging features.Radiographics. 2006; 26:1267–1287.5. Chhabra A, Madhuranthakam AJ, Andreisek G. Magnetic resonance neurography: current perspectives and literature review.Eur Radiol. 2018; 28:698–707.6. Madhuranthakam AJ, Lenkinski RE. Technical advancements in MR neurography. Semin Musculoskelet Radiol. 2015; 19:86–93.
Article7. Chhabra A, Andreisek G, Soldatos T, Wang KC, Flammang AJ, Belzberg AJ, et al. MR neurography: past, present, and future. AJR Am J Roentgenol. 2011; 197:583–591.
Article8. Thawait SK, Chaudhry V, Thawait GK, Wang KC, Belzberg A, Carrino JA, et al. High-resolution MR neurography of diffuse peripheral nerve lesions.AJNR Am J Neuroradiol. 2011; 32:1365–1372.9. Rangavajla G, Mokarram N, Masoodzadehgan N, Pai SB, Bellamkonda RV. Noninvasive imaging of peripheral nerves. Cells Tissues Organs. 2014; 200:69–77.
Article10. Mikityansky I, Zager EL, Yousem DM, Loevner LA. MR Imaging of the brachial plexus.Magn Reson Imaging Clin N Am. 2012; 20:791–826.11. Vargas MI, Gariani J, Delattre BA, Dietemann JL, Lovblad K, Becker M. Three-dimensional MR imaging of the brachial plexus. Semin Musculoskelet Radiol. 2015; 19:137–148.
Article12. Takahara T, Hendrikse J, Yamashita T, Mali WP, Kwee TC, Imai Y, et al. Diffusion-weighted MR neurography of the brachial plexus: feasibility study. Radiology. 2008; 249:653–660.
Article13. Bao H, Wang S, Wang G, Yang L, Hasan MU, Yao B, et al. Diffusion-weighted MR neurography of median and ulnar nerves in the wrist and palm.Eur Radiol. 2017; 27:2359–2366.14. Lacour-Petit MC, Lozeron P, Ducreux D. MRI of peripheral nerve lesions of the lower limbs.Neuroradiology. 2003; 45:166–170.15. Amrami KK, Felmlee JP, Spinner RJ. MRI of peripheral nerves. Neurosurg Clin N Am. 2008; 19:559–572.
Article16. Cho Sims G, Boothe E, Joodi R, Chhabra A. 3D MR neurography of the lumbosacral plexus: obtaining opti-mal images for selective longitudinal nerve depiction.AJNR Am J Neuroradiol. 2016; 37:2158–2162.17. Naraghi A, White LM. Three-dimensional MRI of the musculoskeletal system.AJR Am J Roentgenol. 2012; 199:W283–W293.18. Lell MM, Anders K, Uder M, Klotz E, Ditt H, Vega-Higuera F, et al. New techniques in CT angiography.Radiographics. 2006; 26(Suppl 1):S45–S62.19. Fishman EK, Ney DR, Heath DG, Corl FM, Horton KM, Johnson PT. Volume rendering versus maximum intensity projection in CT angiography: what works best, when, and why.Radiographics. 2006; 26:905–922.20. Mürtz P, Kaschner M, Lakghomi A, Gieseke J, Willinek WA, Schild HH, et al. Diffusion-weighted MR neurography of the brachial and lumbosacral plexus: 3.0 T versus 1.5 T imaging. Eur J Radiol. 2015; 84:696–702.21. Chhabra A, Zhao L, Carrino JA, Trueblood E, Koceski S, Shteriev F, et al. MR neurography: advances.Radiol Res Pract. 2013; 2013:809568.22. Glaser C, D'Anastasi M, Theisen D, Notohamiprodjo M, Horger W, Paul D, et al. Understanding 3D TSE sequences: advantages, disadvantages, and application in MSK imaging. Semin Musculoskelet Radiol. 2015; 19:321–327.
Article23. Chhabra A, Soldatos T, Subhawong TK, Machado AJ, Thawait SK, Wang KC, et al. The application of three-dimensional diffusion-weighted PSIF technique in peripheral nerve imaging of the distal extremities. J Magn Reson Imaging. 2011; 34:962–967.
Article24. Wang X, Harrison C, Mariappan YK, Gopalakrishnan K, Chhabra A, Lenkinski RE, et al. MR neurography of brachial plexus at 3.0 T with robust fat and blood suppression.Radiology. 2017; 283:538–546.25. Kasper JM, Wadhwa V, Scott KM, Rozen S, Xi Y, Chhabra A. SHINKEI–a novel 3D isotropic MR neurography technique: technical advantages over 3DIRTSE-based imaging.Eur Radiol. 2015; 25:1672–1677.26. Chhabra A, Subhawong TK, Bizzell C, Flammang A, Soldatos T. 3T MR neurography using three-dimensional diffusion-weighted PSIF: technical issues and advantages.Skeletal Radiol. 2011; 40:1355–1360.27. Hiwatashi A, Togao O, Yamashita K, Kikuchi K, Ogata H, Yamasaki R, et al. Evaluation of chronic inflammatory demyelinating polyneuropathy: 3D nerve-sheath signal increased with inked rest-tissue rapid acquisition of relaxation enhancement imaging (3D SHINKEI).Eur Radiol. 2017; 27:447–453.28. Bäumer P, Pham M, Ruetters M, Heiland S, Heckel A, Radbruch A, et al. Peripheral neuropathy: detection with diffusion-tensor imaging. Radiology. 2014; 273:185–193.
Article29. Tsuchiya K, Fujikawa A, Tateishi H, Nitatori T. Visualization of cervical nerve roots and their distal nerve fibers by diffusion-weighted scanning using parallel imaging. Acta Radiol. 2006; 47:599–602.
Article30. Zaharchuk G, Saritas EU, Andre JB, Chin CT, Rosenberg J, Brosnan TJ, et al. Reduced field-of-view diffusion imaging of the human spinal cord: comparison with conventional single-shot echo-planar imaging.AJNR Am J Neuroradiol. 2011; 32:813–820.31. Hu J, Li M, Dai Y, Geng C, Tong B, Zhou Z, et al. Combining SENSE and reduced field-of-view for high-resolu-tion diffusion weighted magnetic resonance imaging. Biomed Eng Online. 2018; 17:77.
Article32. Mekle R, Mortamet B, Granziera C, Krueger G, Chevrey N, Theumann N, et al. Magnetization transfer-based 3D visualization of foot peripheral nerves. J Magn Reson Imaging. 2013; 37:1234–1237.
Article33. Bendszus M, Stoll G. Caught in the act: in vivo mapping of macrophage infiltration in nerve injury by magnetic resonance imaging.J Neurosci. 2003; 23:10892–10896.34. Wessig C, Jestaedt L, Sereda MW, Bendszus M, Stoll G. Gadofluorine M-enhanced magnetic resonance nerve imaging: comparison between acute inflammatory and chronic degenerative demyelination in rats. Exp Neurol. 2008; 210:137–143.
Article35. Stoll G, Wessig C, Gold R, Bendszus M. Assessment of lesion evolution in experimental autoimmune neuritis by gadofluorine M-enhanced MR neurography. Exp Neurol. 2006; 197:150–156.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical validation of the 3-dimensional double-echo steady-state with water excitation sequence of MR neurography for preoperative facial and lingual nerve identification
- Frequency and Importance of Nursing Practice between Novice Nurses and Student Nurses
- Magnetic Resonance Neurography with Short Tau Inversion Recovery Sequences for Cervical Radiculopathy
- An Updated Review of Magnetic Resonance Neurography for Plexus Imaging
- Future Supply of and Demand for Diagnostic Radiologists in Korea