Korean J Radiol.
2017 Feb;18(1):71-83. 10.3348/kjr.2017.18.1.71.
T-Cell Non-Hodgkin Lymphomas: Spectrum of Disease and the Role of Imaging in the Management of Common Subtypes
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea. hesoni86@gmail.com
- 2Department of Imaging, Dana Farber Cancer Institute, Harvard Medical School, Boston, MA 02215, USA.
- 3Department of Radiology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115, USA.
- KMID: 2468123
- DOI: http://doi.org/10.3348/kjr.2017.18.1.71
Abstract
- T-cell non-Hodgkin lymphomas (NHLs) are biologically diverse, uncommon malignancies characterized by a spectrum of imaging findings according to subtype. The purpose of this review is to describe the common subtypes of T-cell NHL, highlight important differences between cutaneous, various peripheral and precursor subtypes, and summarize imaging features and the role of imaging in the management of this diverse set of diseases.
Keyword
MeSH Terms
-
Female
Humans
Lymphoma, Extranodal NK-T-Cell/diagnostic imaging
Lymphoma, Large-Cell, Anaplastic/diagnostic imaging
Lymphoma, T-Cell/*diagnostic imaging
Lymphoma, T-Cell, Cutaneous/diagnostic imaging
Lymphoma, T-Cell, Peripheral/diagnostic imaging
Magnetic Resonance Imaging
Male
Positron Emission Tomography Computed Tomography
Precursor Cell Lymphoblastic Leukemia-Lymphoma/diagnostic imaging
Tomography, X-Ray Computed
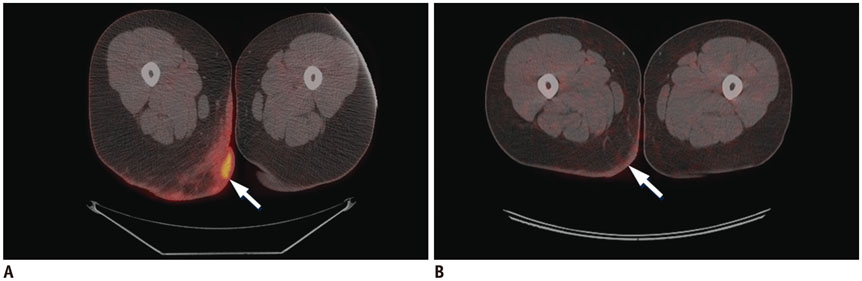
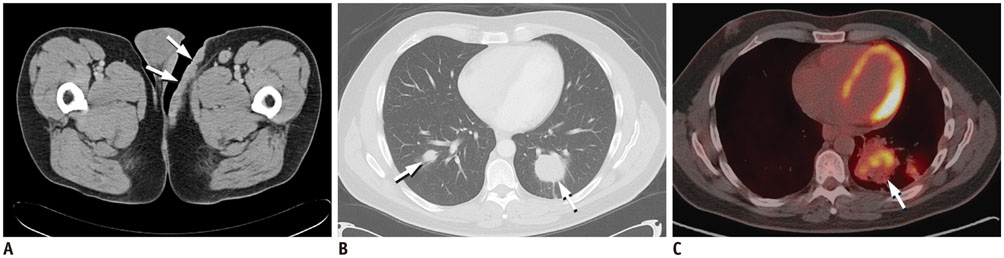
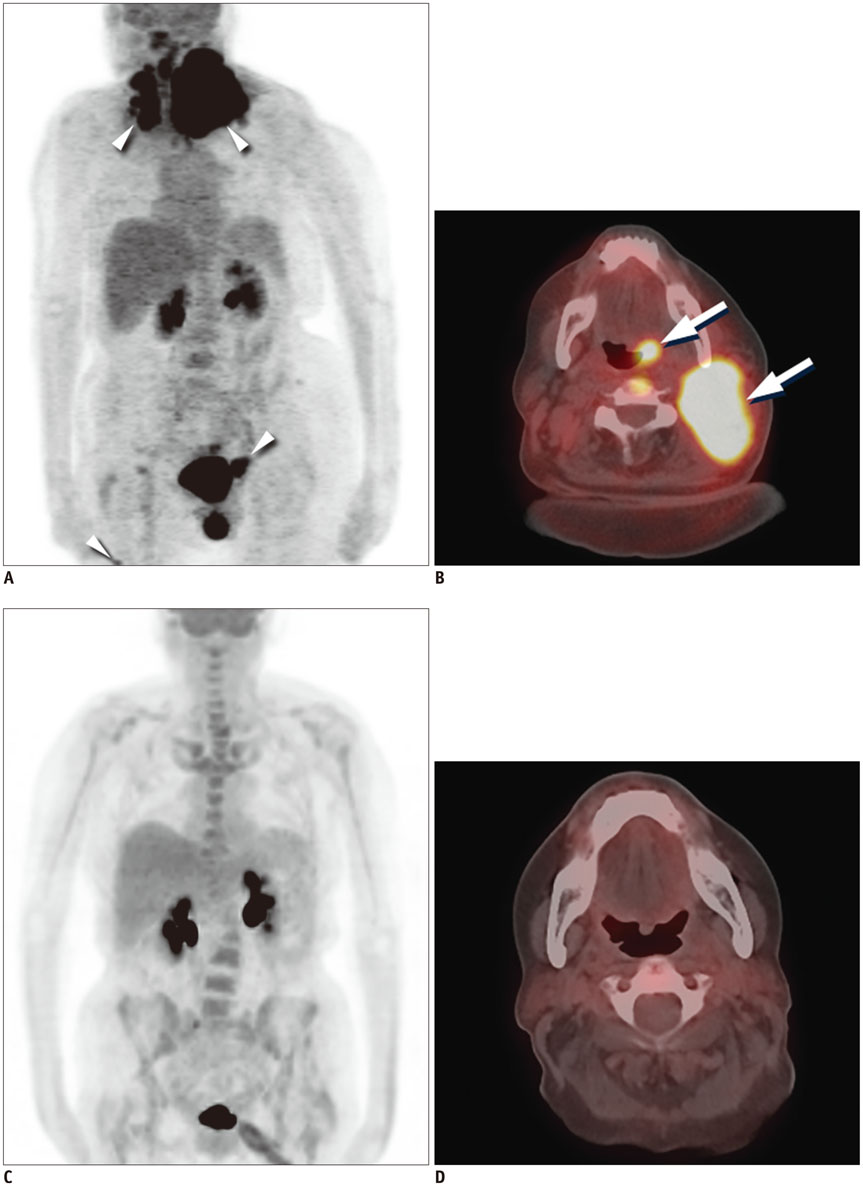
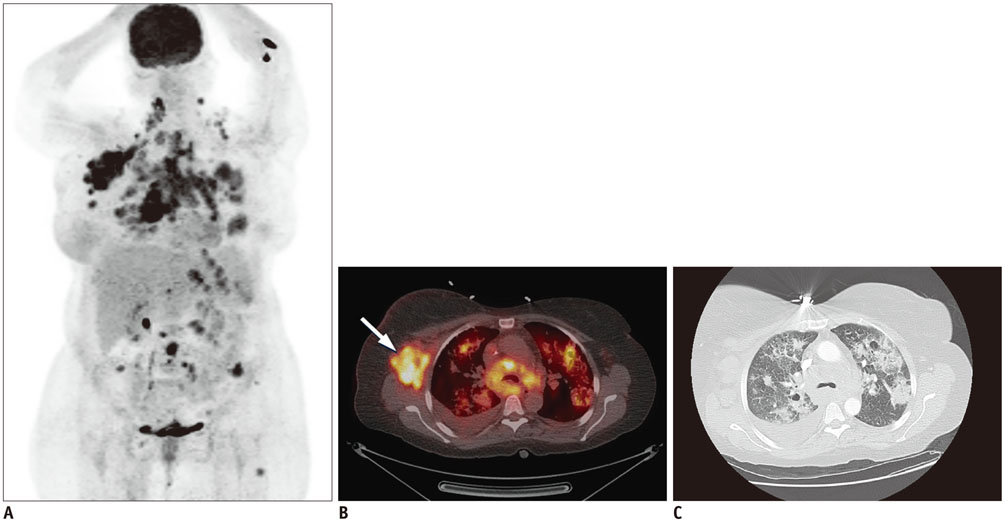
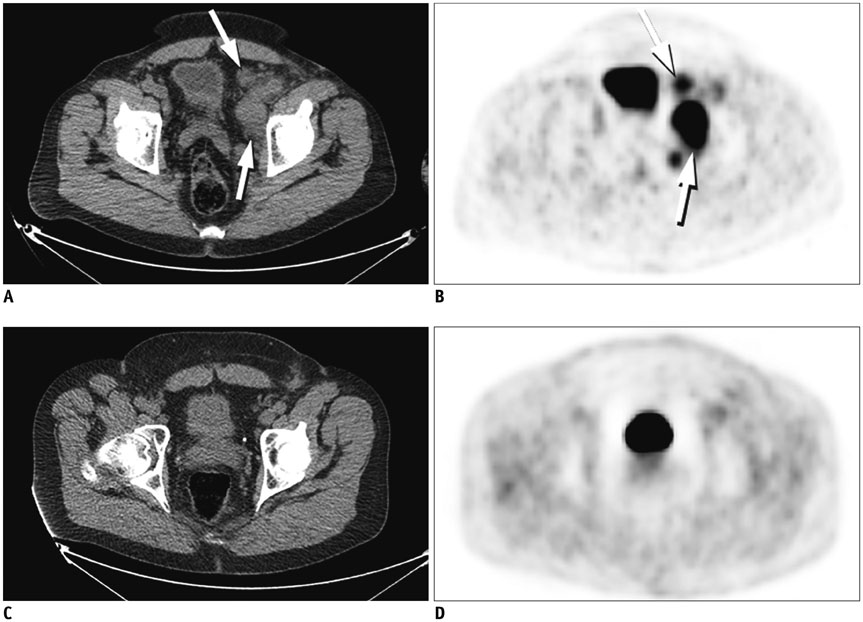
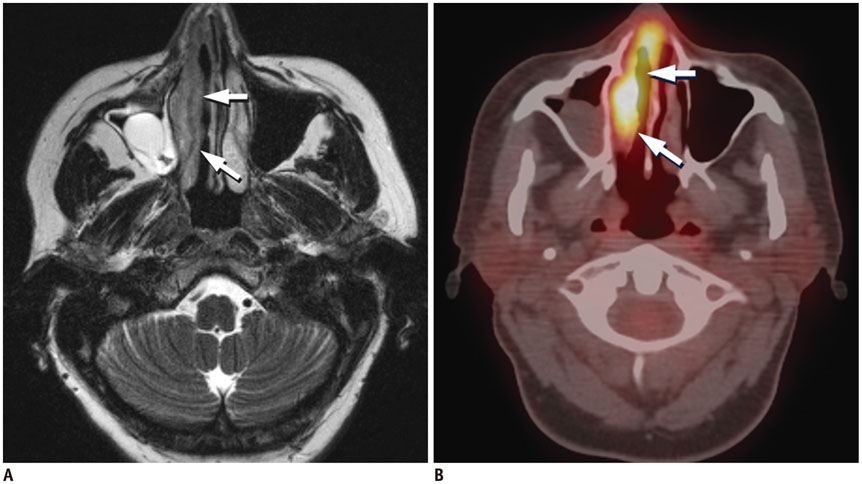
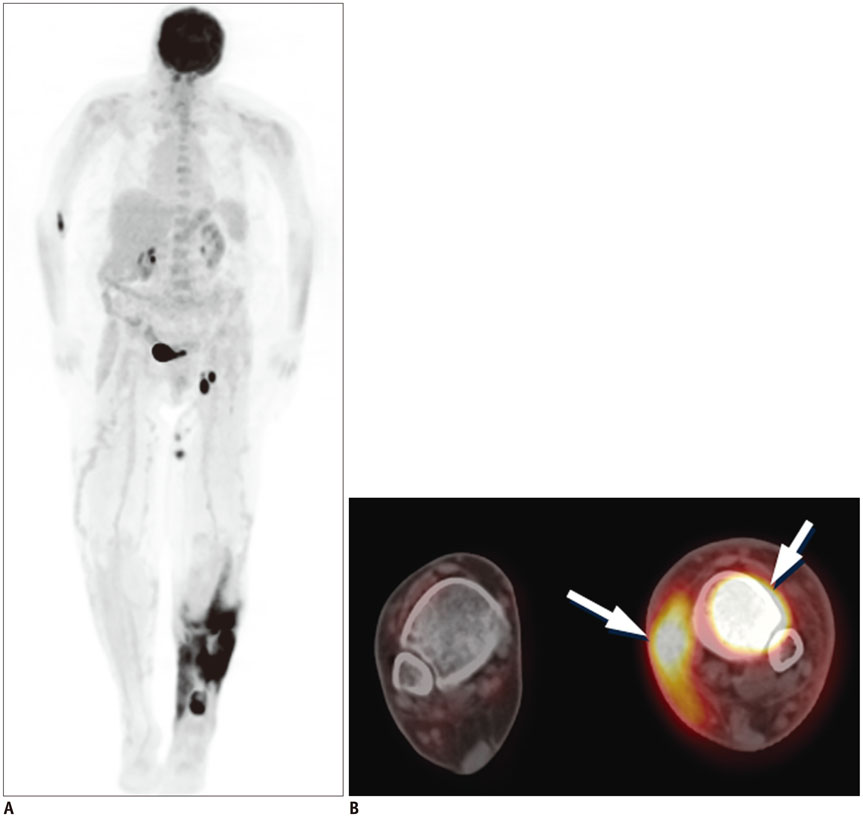
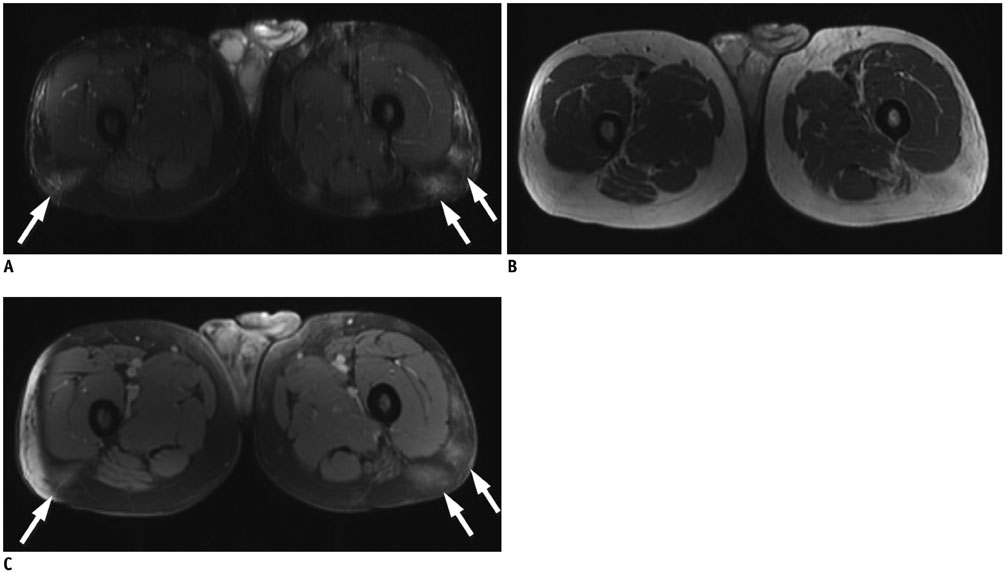
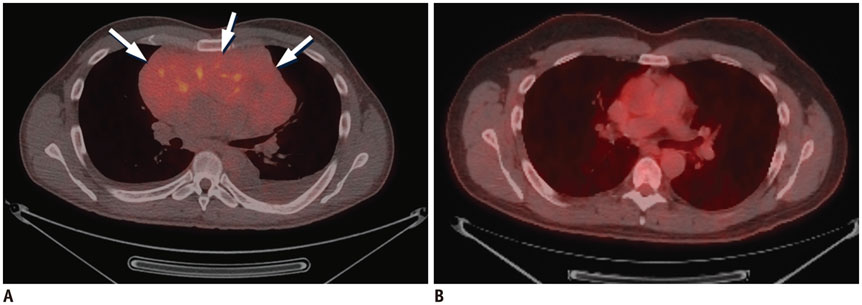
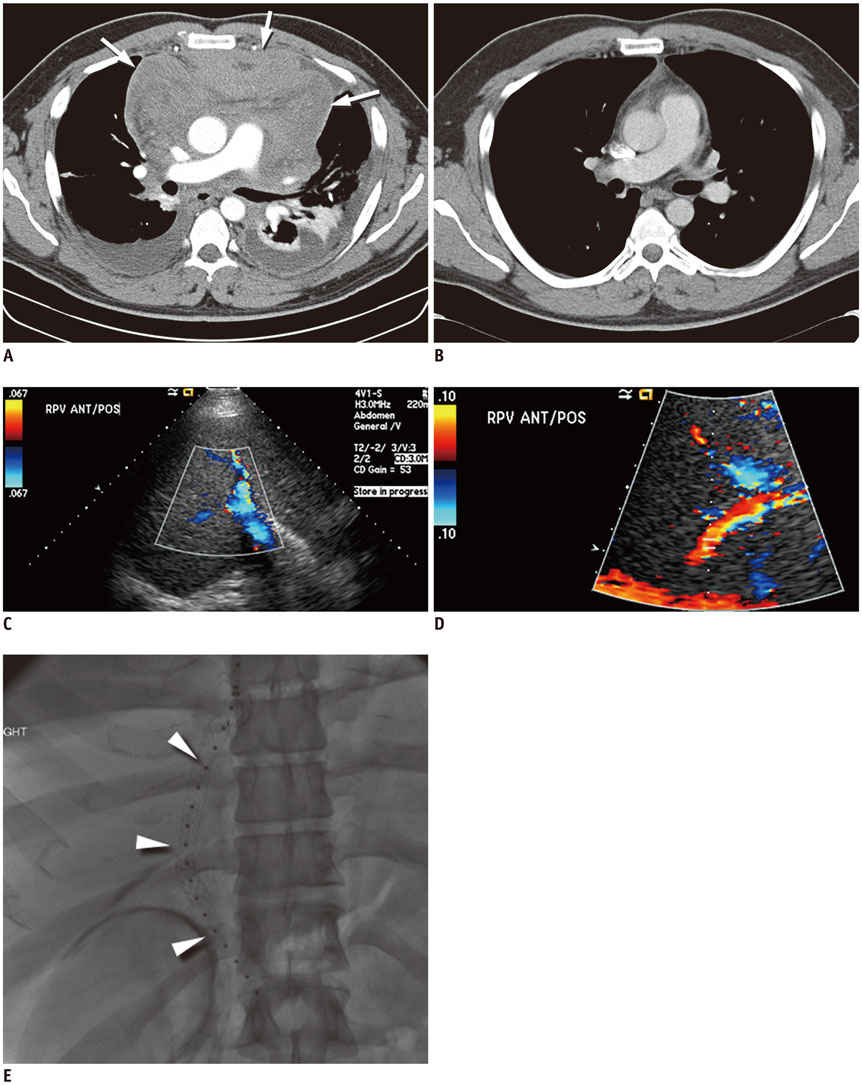
Figure
Cited by 1 articles
-
A Case of Extranodal Natural Killer/T Cell Lymphoma Combined With Actinomycosis
Jun Seop Kim, Tae Hoon An, Nam-Kyung Yeo
Korean J Otorhinolaryngol-Head Neck Surg. 2022;65(11):727-733. doi: 10.3342/kjorl-hns.2022.00703.
Reference
-
1. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997; 89:3909–3918.2. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127:2375–2390.3. Vose J, Armitage J, Weisenburger D. International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008; 26:4124–4130.4. Abouyabis AN, Shenoy PJ, Lechowicz MJ, Flowers CR. Incidence and outcomes of the peripheral T-cell lymphoma subtypes in the United States. Leuk Lymphoma. 2008; 49:2099–2107.5. Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006; 107:265–276.6. Vinnicombe SJ, Reznek RH. Computerised tomography in the staging of Hodgkin's disease and non-Hodgkin's lymphoma. Eur J Nucl Med Mol Imaging. 2003; 30:Suppl 1. S42–S55.7. Raanani P, Shasha Y, Perry C, Metser U, Naparstek E, Apter S, et al. Is CT scan still necessary for staging in Hodgkin and non-Hodgkin lymphoma patients in the PET/CT era? Ann Oncol. 2006; 17:117–122.8. Cheson BD. Role of functional imaging in the management of lymphoma. J Clin Oncol. 2011; 29:1844–1854.9. Feeney J, Horwitz S, Gönen M, Schöder H. Characterization of T-cell lymphomas by FDG PET/CT. AJR Am J Roentgenol. 2010; 195:333–340.10. Tatsumi M, Cohade C, Nakamoto Y, Fishman EK, Wahl RL. Direct comparison of FDG PET and CT findings in patients with lymphoma: initial experience. Radiology. 2005; 237:1038–1045.11. Cronin CG, Swords R, Truong MT, Viswanathan C, Rohren E, Giles FJ, et al. Clinical utility of PET/CT in lymphoma. AJR Am J Roentgenol. 2010; 194:W91–W103.12. Moskowitz CH, Schöder H. Current status of the role of PET imaging in diffuse large B-cell lymphoma. Semin Hematol. 2015; 52:138–142.13. Gill H, Liang RH, Tse E. Extranodal natural-killer/T-cell lymphoma, nasal type. Adv Hematol. 2010; 2010:627401.14. Levine BD, Seeger LL, James AW, Motamedi K. Subcutaneous panniculitis-like T-cell lymphoma: MRI features and literature review. Skeletal Radiol. 2014; 43:1307–1311.15. Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007; 143:854–859.16. National Cancer Institute. PDQ® adult treatment editorial board. PDQ mycosis fungoides and the Sézary syndrome treatment. Web site. Accessed June 28, 2016. http://www.cancer.gov/types/lymphoma/hp/mycosis-fungoides-treatment-pdq.17. Siegel RS, Pandolfino T, Guitart J, Rosen S, Kuzel TM. Primary cutaneous T-cell lymphoma: review and current concepts. J Clin Oncol. 2000; 18:2908–2925.18. Kuo PH, McClennan BL, Carlson K, Wilson LD, Edelson RL, Heald PW, et al. FDG-PET/CT in the evaluation of cutaneous T-cell lymphoma. Mol Imaging Biol. 2008; 10:74–81.19. Olsen EA, Whittaker S, Kim YH, Duvic M, Prince HM, Lessin SR, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011; 29:2598–2607.20. Jhanwar YS, Straus DJ. The role of PET in lymphoma. J Nucl Med. 2006; 47:1326–1334.21. Ueda T, Hosoki N, Isobe K, Yamamoto S, Motoori K, Shinkai H, et al. Diffuse pulmonary involvement by mycosis fungoides: high-resolution computed tomography and pathologic findings. J Thorac Imaging. 2002; 17:157–159.22. Baser S, Onn A, Lin E, Morice RC, Duvic M. Pulmonary manifestations in patients with cutaneous T-cell lymphomas. Cancer. 2007; 109:1550–1555.23. Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003; 139:857–866.24. Spaccarelli N, Gharavi M, Saboury B, Cheng G, Rook AH, Alavi A. Role of (18)F-fluorodeoxyglucose positron emission tomography imaging in the management of primary cutaneous lymphomas. Hell J Nucl Med. 2014; 17:78–84.25. Kim JS, Jeong YJ, Sohn MH, Lim ST, Kim DW, Jeong HJ, et al. Before and after treatment 18F-FDG PET/CT images in a patient with cutaneous T-cell lymphoma. Eur J Nucl Med Mol Imaging. 2010; 37:1995.26. Damsky WE, Choi J. Genetics of cutaneous T cell Lymphoma: from bench to bedside. Curr Treat Options Oncol. 2016; 17:33.27. Vergier B, de Muret A, Beylot-Barry M, Vaillant L, Ekouevi D, Chene G, et al. French Study Group of Cutaneious Lymphomas. Transformation of mycosis fungoides: clinicopathological and prognostic features of 45 cases. Blood. 2000; 95:2212–2218.28. Salhany KE, Cousar JB, Greer JP, Casey TT, Fields JP, Collins RD. Transformation of cutaneous T cell lymphoma to large cell lymphoma. A clinicopathologic and immunologic study. Am J Pathol. 1988; 132:265–277.29. Herrmann JL, Hughey LC. Recognizing large-cell transformation of mycosis fungoides. J Am Acad Dermatol. 2012; 67:665–672.30. Diamandidou E, Colome-Grimmer M, Fayad L, Duvic M, Kurzrock R. Transformation of mycosis fungoides/Sezary syndrome: clinical characteristics and prognosis. Blood. 1998; 92:1150–1159.31. Chott A, Dragosics B, Radaszkiewicz T. Peripheral T-cell lymphomas of the intestine. Am J Pathol. 1992; 141:1361–1371.32. Bekkenk MW, Vermeer MH, Jansen PM, van Marion AM, Canninga-van Dijk MR, Kluin PM, et al. Peripheral T-cell lymphomas unspecified presenting in the skin: analysis of prognostic factors in a group of 82 patients. Blood. 2003; 102:2213–2219.33. Weisenburger DD, Savage KJ, Harris NL, Gascoyne RD, Jaffe ES, MacLennan KA, et al. Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood. 2011; 117:3402–3408.34. Savage KJ, Ferreri AJ, Zinzani PL, Pileri SA. Peripheral T-cell lymphoma--not otherwise specified. Crit Rev Oncol Hematol. 2011; 79:321–329.35. Vose JM. Peripheral T-cell non-Hodgkin's lymphoma. Hematol Oncol Clin North Am. 2008; 22:997–1005. x36. Wang T, Feldman AL, Wada DA, Lu Y, Polk A, Briski R, et al. GATA-3 expression identifies a high-risk subset of PTCL, NOS with distinct molecular and clinical features. Blood. 2014; 123:3007–3015.37. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014; 32:3059–3068.38. Shustov AR, Savage KJ. Does high-dose therapy and autologous hematopoietic stem cell transplantation have a role in the primary treatment of peripheral T-cell lymphomas? ASH evidence-based review 2008. Hematology Am Soc Hematol Educ Program. 2008; 39–41.39. Gallamini A, Stelitano C, Calvi R, Bellei M, Mattei D, Vitolo U, et al. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004; 103:2474–2479.40. Dupuis J, Emile JF, Mounier N, Gisselbrecht C, Martin-Garcia N, Petrella T, et al. Prognostic significance of Epstein-Barr virus in nodal peripheral T-cell lymphoma, unspecified: A Groupe d'Etude des Lymphomes de l'Adulte (GELA) study. Blood. 2006; 108:4163–4169.41. Weiler-Sagie M, Bushelev O, Epelbaum R, Dann EJ, Haim N, Avivi I, et al. (18)F-FDG avidity in lymphoma readdressed: a study of 766 patients. J Nucl Med. 2010; 51:25–30.42. Federico M, Rudiger T, Bellei M, Nathwani BN, Luminari S, Coiffier B, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013; 31:240–246.43. Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008; 111:5496–5504.44. Parrilla Castellar ER, Jaffe ES, Said JW, Swerdlow SH, Ketterling RP, Knudson RA, et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood. 2014; 124:1473–1480.45. Lee DY, Lee JJ, Kim JY, Park SH, Chae SY, Kim S, et al. (18)F-FDG PET in patients with primary systemic anaplastic large cell lymphoma: differential features according to expression of anaplastic lymphoma kinase. Nucl Med Mol Imaging. 2013; 47:249–256.46. Panwalkar AW, Armitage JO. T-cell/NK-cell lymphomas: a review. Cancer Lett. 2007; 253:1–13.47. Au WY, Weisenburger DD, Intragumtornchai T, Nakamura S, Kim WS, Sng I, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009; 113:3931–3937.48. Ahn HK, Suh C, Chuang SS, Suzumiya J, Ko YH, Kim SJ, et al. Extranodal natural killer/T-cell lymphoma from skin or soft tissue: suggestion of treatment from multinational retrospective analysis. Ann Oncol. 2012; 23:2703–2707.49. Ou CH, Chen CC, Ling JC, Chai JW, Wu CH, Chen WH, et al. Nasal NK/T-cell lymphoma: computed tomography and magnetic resonance imaging findings. J Chin Med Assoc. 2007; 70:207–212.50. Zhou X, Lu K, Geng L, Li X, Jiang Y, Wang X. Utility of PET/CT in the diagnosis and staging of extranodal natural killer/T-cell lymphoma: a systematic review and meta-analysis. Medicine (Baltimore). 2014; 93:e258.51. Wu HB, Wang QS, Wang MF, Li HS, Zhou WL, Ye XH, et al. Utility of 18F-FDG PET/CT for staging NK/T-cell lymphomas. Nucl Med Commun. 2010; 31:195–200.52. Li YX, Fang H, Liu QF, Lu J, Qi SN, Wang H, et al. Clinical features and treatment outcome of nasal-type NK/T-cell lymphoma of Waldeyer ring. Blood. 2008; 112:3057–3064.53. Li YX, Yao B, Jin J, Wang WH, Liu YP, Song YW, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. 2006; 24:181–189.54. Pan ZH, Huang HQ, Lin XB, Xia YF, Xia ZJ, Peng YL, et al. [Prognostic analysis of patients with nasal-type NK/T-cell non-Hodgkin's lymphoma--a report of 93 cases]. Ai Zheng. 2005; 24:1493–1497.55. Kim GE, Lee SW, Chang SK, Park HC, Pyo HR, Kim JH, et al. Combined chemotherapy and radiation versus radiation alone in the management of localized angiocentric lymphoma of the head and neck. Radiother Oncol. 2001; 61:261–269.56. Li CC, Tien HF, Tang JL, Yao M, Chen YC, Su IJ, et al. Treatment outcome and pattern of failure in 77 patients with sinonasal natural killer/T-cell or T-cell lymphoma. Cancer. 2004; 100:366–375.57. Willemze R, Jansen PM, Cerroni L, Berti E, Santucci M, Assaf C, et al. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008; 111:838–845.58. Jeong SI, Lim HS, Choi YR, Kim JW, Park MH, Cho JS, et al. Subcutaneous panniculitis-like T-cell lymphoma of the breast. Korean J Radiol. 2013; 14:391–394.59. Papajík T, Mysliveček M, Sedová Z, Buriánková E, Procházka V, Koranda P, et al. Standardised uptake value of 18F-FDG on staging PET/CT in newly diagnosed patients with different subtypes of non-Hodgkin’s lymphoma. Eur J Haematol. 2011; 86:32–37.60. Lim GY, Hahn ST, Chung NG, Kim HK. Subcutaneous panniculitis-like T-cell lymphoma in a child: whole-body MRI in the initial and follow-up evaluations. Pediatr Radiol. 2009; 39:57–61.61. Hoelzer D, Gökbuget N, Digel W, Faak T, Kneba M, Reutzel R, et al. Outcome of adult patients with T-lymphoblastic lymphoma treated according to protocols for acute lymphoblastic leukemia. Blood. 2002; 99:4379–4385.62. Otero HJ, Jagannathan JP, Prevedello LM, Johnston CJ, Ramaiya NH, Van den Abbeele AD, et al. CT and PET/CT findings of T-cell lymphoma. AJR Am J Roentgenol. 2009; 193:349–358.63. Morel P, Lepage E, Brice P, Dupriez B, D'Agay MF, Fenaux P, et al. Prognosis and treatment of lymphoblastic lymphoma in adults: a report on 80 patients. J Clin Oncol. 1992; 10:1078–1085.64. Cortelazzo S, Ponzoni M, Ferreri AJ, Hoelzer D. Lymphoblastic lymphoma. Crit Rev Oncol Hematol. 2011; 79:330–343.65. Gökbuget N, Wolf A, Stelljes M, Hüttmann A, Buss EC, Viardot A, et al. Favorable outcome in a large cohort of prospectively treated adult patients with T-lmphoblastic lymphoma (T-LBL) despite slowly evolving complete remission assessed by conventional radiography. Blood. 2014; 124:370.66. Lepretre S, Touzart A, Vermeulin T, Picquenot JM, Tanguy-Schmidt A, Salles G, et al. Pediatric-like acute lymphoblastic leukemia therapy in adults with lymphoblastic lymphoma: the GRAALL-LYSA LL03 study. J Clin Oncol. 2016; 34:572–580.67. Aljurf M, Zaidi SZ. Chemotherapy and hematopoietic stem cell transplantation for adult T-cell lymphoblastic lymphoma: current status and controversies. Biol Blood Marrow Transplant. 2005; 11:739–754.68. Kaiser U, Uebelacker I, Havemann K. Non-Hodgkin's lymphoma protocols in the treatment of patients with Burkitt's lymphoma and lymphoblastic lymphoma: a report on 58 patients. Leuk Lymphoma. 1999; 36:101–108.69. Coleman CN, Picozzi VJ Jr, Cox RS, McWhirter K, Weiss LM, Cohen JR, et al. Treatment of lymphoblastic lymphoma in adults. J Clin Oncol. 1986; 4:1628–1637.70. Slater DE, Mertelsmann R, Koziner B, Higgins C, McKenzie S, Schauer P, et al. Lymphoblastic lymphoma in adults. J Clin Oncol. 1986; 4:57–67.71. Song KW, Barnett MJ, Gascoyne RD, Chhanabhai M, Forrest DL, Hogge DE, et al. Primary therapy for adults with T-cell lymphoblastic lymphoma with hematopoietic stem-cell transplantation results in favorable outcomes. Ann Oncol. 2007; 18:535–540.72. Mohty B, Mohty M. Long-term complications and side effects after allogeneic hematopoietic stem cell transplantation: an update. Blood Cancer J. 2011; 1:e16.73. Afessa B, Peters SG. Major complications following hematopoietic stem cell transplantation. Semin Respir Crit Care Med. 2006; 27:297–309.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CD30 (Ber H2) Distribution in Hodgkin's Disease and non-Hodgkin's Lymphoma
- The Incidence of the Epstein-Barr Virus Nuclear Antigen in Non-Hodgkin's Lymphomas of the Gastrointestinal Tract
- Malignant lymphomas in Korea
- EBV in situ hybridization study for non-Hodgkin's lymphomas
- Immunoreactivity of CD99 in Non-Hodgkin's Lymphoma: Unexpected Frequent Expression in ALK-positive Anaplastic Large Cell Lymphoma