Investig Clin Urol.
2020 Jan;61(1):42-50. 10.4111/icu.2020.61.1.42.
Preoperative prostate health index and %p2PSA as the significant biomarkers of postoperative pathological outcomes of prostate cancer in Korean males: A prospective multi-institutional study
- Affiliations
-
- 1Department of Urology, Kangwon National University School of Medicine, Chuncheon, Korea. Urodr348@kangwon.ac.kr
- 2Department of Urology, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 3Department of Urology, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea.
- 4Department of Urology, Hallym University Chuncheon Sacred Heart Hospital, Hallym University College of Medicine, Chuncheon, Korea.
- KMID: 2466791
- DOI: http://doi.org/10.4111/icu.2020.61.1.42
Abstract
- PURPOSE
To evaluate the clinical utility of percentage of serum prostate-specific antigen (proPSA) to free PSA (%p2PSA) and the prostate health index (PHI) for predicting aggressive pathological outcomes of radical prostatectomy (RP) in Korean males.
MATERIALS AND METHODS
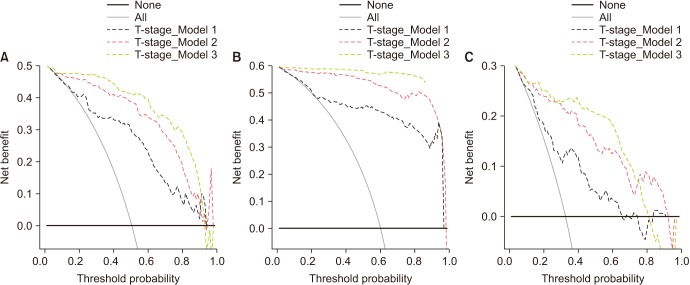
This prospective observational multicenter study included 160 Korean males who consecutively underwent RP. The predictive utility of preoperative %p2PSA and PHI for predicting the following pathological outcomes of RP including pT3 disease, pathologic Gleason sum ≥7, and Gleason sum upgrading was investigated using multivariate and decision-curve analyses.
RESULTS
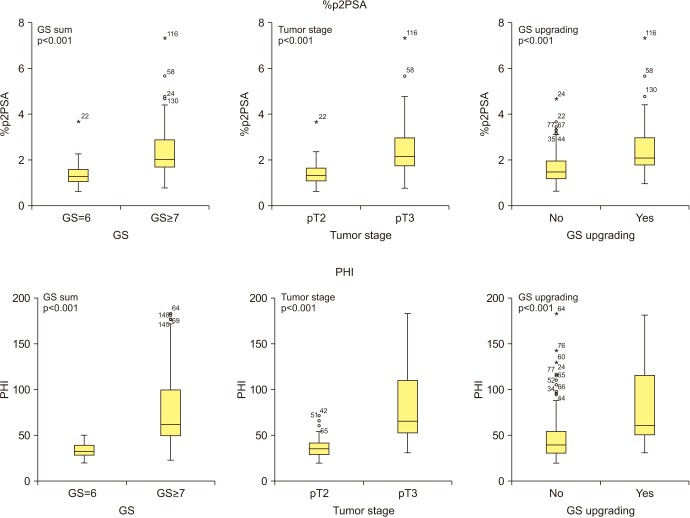
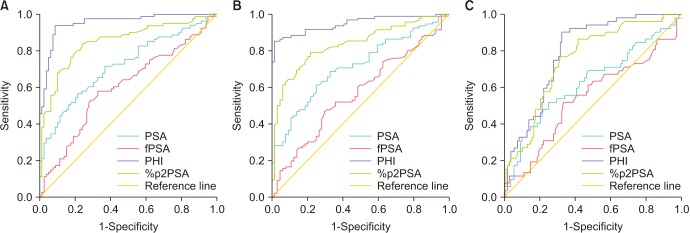
The PHI and %p2PSA levels were significantly higher in patients with pT3 disease, pathologic Gleason sum ≥7, and Gleason sum upgrading. On univariate analysis, PHI was an accurate predictor of pT3 disease, pathologic Gleason sum ≥7, and Gleason sum upgrading. Multivariate and decision curve analyses revealed that inclusion of PHI to a base multivariate model including total PSA, percentage free PSA, PSA density, percentage of positive biopsy core, biopsy Gleason sum, and clinical stage factors significantly increased its predictive accuracy; %p2PSA showed a similar result. However, PHI was a more valuable predictor of pathological outcomes of RP.
CONCLUSIONS
This study revealed PHI and %p2PSA as preoperative biomarkers of pathological outcomes in Korean males who underwent RP for prostate cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Hugosson J, Carlsson S, Aus G, Bergdahl S, Khatami A, Lodding P, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010; 11:725–732. PMID: 20598634.
Article2. McGrath S, Christidis D, Perera M, Hong SK, Manning T, Vela I, et al. Prostate cancer biomarkers: are we hitting the mark? Prostate Int. 2016; 4:130–135. PMID: 27995111.
Article3. Moyer VA. U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012; 157:120–134. PMID: 22801674.
Article4. Hori S, Blanchet JS, McLoughlin J. From prostate-specific antigen (PSA) to precursor PSA (proPSA) isoforms: a review of the emerging role of proPSAs in the detection and management of early prostate cancer. BJU Int. 2013; 112:717–728. PMID: 22759214.
Article5. Hendriks RJ, van Oort IM, Schalken JA. Blood-based and urinary prostate cancer biomarkers: a review and comparison of novel biomarkers for detection and treatment decisions. Prostate Cancer Prostatic Dis. 2017; 20:12–19. PMID: 27922627.
Article6. Sriplakich S, Lojanapiwat B, Chongruksut W, Phuriyaphan S, Kitirattakarn P, Jun-Ou J, et al. Prospective performance of the Prostate Health Index in prostate cancer detection in the first prostate biopsy of men with a total prostatic specific antigen of 4-10 ng/mL and negative digital rectal examination. Prostate Int. 2018; 6:136–139. PMID: 30505815.7. Jansen FH, van Schaik RH, Kurstjens J, Horninger W, Klocker H, Bektic J, et al. Prostate-specific antigen (PSA) isoform p2PSA in combination with total PSA and free PSA improves diagnostic accuracy in prostate cancer detection. Eur Urol. 2010; 57:921–927. PMID: 20189711.
Article8. Guazzoni G, Nava L, Lazzeri M, Scattoni V, Lughezzani G, Maccagnano C, et al. Prostate-specific antigen (PSA) isoform p2PSA significantly improves the prediction of prostate cancer at initial extended prostate biopsies in patients with total PSA between 2.0 and 10 ng/ml: results of a prospective study in a clinical setting. Eur Urol. 2011; 60:214–222. PMID: 21482022.
Article9. Eminaga O, Bögemann M, Breil B, Titze U, Wötzel F, Eltze E, et al. Preoperative prostate-specific antigen isoform p2PSA ≤ 22.5 pg/ml predicts advanced prostate cancer in patients undergoing radical prostatectomy. Urol Oncol. 2014; 32:1317–1326. PMID: 24893699.10. Fossati N, Buffi NM, Haese A, Stephan C, Larcher A, McNicholas T, et al. Preoperative prostate-specific antigen isoform p2PSA and its derivatives, %p2PSA and prostate health index, predict pathologic outcomes in patients undergoing radical prostatectomy for prostate cancer: results from a Multicentric European Prospective Study. Eur Urol. 2015; 68:132–138. PMID: 25139197.
Article11. Guazzoni G, Lazzeri M, Nava L, Lughezzani G, Larcher A, Scattoni V, et al. Preoperative prostate-specific antigen isoform p2PSA and its derivatives, %p2PSA and prostate health index, predict pathologic outcomes in patients undergoing radical prostatectomy for prostate cancer. Eur Urol. 2012; 61:455–466. PMID: 22078333.
Article12. Chiu PK, Ng CF, Semjonow A, Zhu Y, Vincendeau S, Houlgatte A, et al. A multicentre evaluation of the role of the Prostate Health Index (PHI) in regions with differing prevalence of prostate cancer: adjustment of PHI reference ranges is needed for European and Asian settings. Eur Urol. 2019; 75:558–561. PMID: 30396635.
Article13. Tang B, Han CT, Lu XL, Wan FN, Zhang CZ, Zhu Y, et al. Preoperative prostate health index predicts poor pathologic outcomes of radical prostatectomy in patients with biopsy-detected low-risk patients prostate cancer: results from a Chinese prospective cohort. Prostate Cancer Prostatic Dis. 2018; 21:64–70. PMID: 29213105.
Article14. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386. PMID: 25220842.
Article15. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127:2893–2917. PMID: 21351269.
Article16. Ha Chung B, Horie S, Chiong E. The incidence, mortality, and risk factors of prostate cancer in Asian men. Prostate Int. 2019; 7:1–8. PMID: 30937291.
Article17. Tseng CH. Prostate cancer mortality in Taiwanese men: increasing age-standardized trend in general population and increased risk in diabetic men. Ann Med. 2011; 43:142–150. PMID: 21284526.
Article18. Park SK, Sakoda LC, Kang D, Chokkalingam AP, Lee E, Shin HR, et al. Rising prostate cancer rates in South Korea. Prostate. 2006; 66:1285–1291. PMID: 16741923.
Article19. Chen C, Naidoo N, Yang Q, Hartman M, Verkooijen HM, Loy EY, et al. A comparative population-based study of prostate cancer incidence and mortality rates in Singapore, Sweden and Geneva, Switzerland from 1973 to 2006. BMC Cancer. 2012; 12:222. PMID: 22673095.20. Katanoda K, Hori M, Matsuda T, Shibata A, Nishino Y, Hattori M, et al. An updated report on the trends in cancer incidence and mortality in Japan, 1958-2013. Jpn J Clin Oncol. 2015; 45:390–401. PMID: 25637502.
Article21. Park H, Lee SW, Song G, Kang TW, Jung JH, Chung HC, et al. Diagnostic performance of %[−2]proPSA and Prostate Health Index for prostate cancer: prospective, multi-institutional study. J Korean Med Sci. 2018; 33:e94. PMID: 29495138.
Article22. van der Kwast TH, Amin MB, Billis A, Epstein JI, Griffiths D, Humphrey PA, et al. ISUP Prostate Cancer Group. International Society of Urological Pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens. Working group 2: T2 substaging and prostate cancer volume. Mod Pathol. 2011; 24:16–25. PMID: 20818340.
Article23. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL. ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) consensus conference on Gleason Grading of prostatic carcinoma. Am J Surg Pathol. 2005; 29:1228–1242. PMID: 16096414.
Article24. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845. PMID: 3203132.
Article25. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006; 26:565–574. PMID: 17099194.
Article26. Chiu PK, Lai FM, Teoh JY, Lee WM, Yee CH, Chan ES, et al. Prostate Health Index and %p2PSA predict aggressive prostate cancer pathology in Chinese patients undergoing radical prostatectomy. Ann Surg Oncol. 2016; 23:2707–2714. PMID: 26965697.
Article27. Fossati N, Lazzeri M, Haese A, McNicholas T, de la Taille A, Buffi NM, et al. Clinical performance of serum isoform [−2]proPSA (p2PSA), and its derivatives %p2PSA and the Prostate Health Index, in men aged <60 years: results from a multicentric European study. BJU Int. 2015; 115:913–920. PMID: 24589357.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnostic Performance of %[-2]proPSA and Prostate Health Index for Prostate Cancer: Prospective, Multi-institutional Study
- Clinical Use of [-2]proPSA (p2PSA) and Its Derivatives (%p2PSA and Prostate Health Index) for the Detection of Prostate Cancer: A Review of the Literature
- Potential Utility of Prostate Health Index Density for Prostate Cancer Detection and Prediction in Korean Men: A Prospective Multicenter Study
- Nomogram Using Prostate Health Index for Predicting Prostate Cancer in the Gray Zone: Prospective, Multicenter Study
- Beyond Prostate Specific Antigen: New Prostate Cancer Screening Options