Neonatal Med.
2019 Nov;26(4):204-212. 10.5385/nm.2019.26.4.204.
Risk Factors for Delayed Hyperthyrotropinemia in Late Preterm Infants
- Affiliations
-
- 1Department of Pediatrics, Pusan National University Children's Hospital, Pusan National University School of Medicine, Yangsan, Korea. skybluehym@gmail.com
- 2Department of Pediatrics, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea.
- KMID: 2466640
- DOI: http://doi.org/10.5385/nm.2019.26.4.204
Abstract
- PURPOSE
This study aimed to investigate the prevalence of delayed thyroid dysfunction based on iodine disinfectant use and to analyze associated risk factors.
METHODS
A retrospective study was conducted on late preterm infants admitted to the neonatal intensive care unit between January 2010 and June 2018, who underwent neonatal thyroid screening (NTS) and ≥2 thyroid function tests (TFTs). NTS was performed 3 days after birth, with at least two TFTs 1 week and 2 to 4 weeks after birth. To distinguish between normal and dysfunctional thyroid levels, we reviewed TFT results at 2 to 4 weeks and examined possible risk factors for the development of thyroid dysfunction.
RESULTS
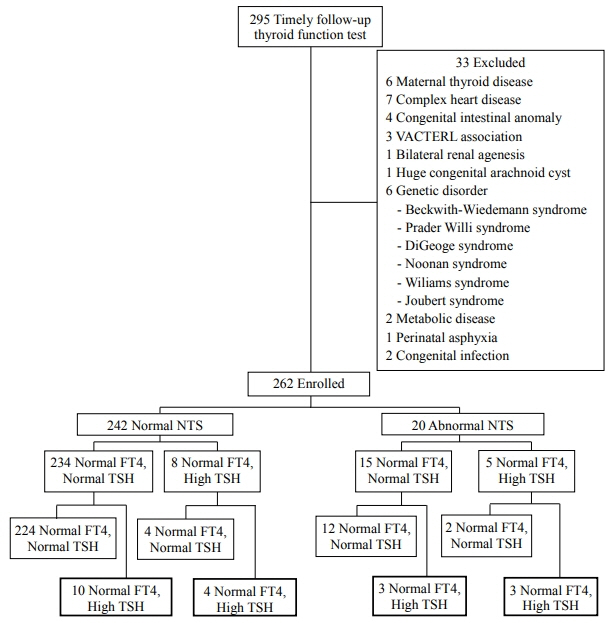
Of 295 late preterm infants, 262 were enrolled with a mean gestational age and birth weight of 34.8±0.7 weeks and 2,170±454 g, respectively. A total of 7.6% developed hyperthyrotropinemia at the age of 24.3±14.6 days (range, 12 to 69). The incidence of hyperthyrotropinemia during iodine use was approximately 12.6%, while that during discontinuation was 2.4% (P=0.002). Multivariate analysis revealed that small for gestational age (SGA), iodine disinfectant use, and abnormal NTS results were significant risk factors for delayed hyperthyrotropinemia (adjusted odds ratio [AOR]: 4.27, P=0.008; AOR: 8.24, P=0.003; and AOR: 7.80, P=0.002, respectively).
CONCLUSION
Delayed hyperthyrotropinemia was prevalent in late preterm infants exposed to topical iodine and those identified as being SGA. Secondary TFTs should be considered 2 to 4 weeks after birth for this population at risk.
Keyword
MeSH Terms
-
Birth Weight
Congenital Hypothyroidism
Gestational Age
Humans
Incidence
Infant, Newborn
Infant, Premature*
Intensive Care, Neonatal
Iodine
Mass Screening
Multivariate Analysis
Odds Ratio
Parturition
Population Characteristics
Prevalence
Retrospective Studies
Risk Factors*
Thyroid Function Tests
Thyroid Gland
Thyrotropin
Iodine
Thyrotropin
Figure
Reference
-
1. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012; 379:2162–72.2. Woythaler M. Neurodevelopmental outcomes of the late preterm infant. Semin Fetal Neonatal Med. 2019; 24:54–9.3. Raju TN, Higgins RD, Stark AR, Leveno KJ. Optimizing care and outcome for late-preterm (near-term) infants: a summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics. 2006; 118:1207–14.4. Kugelman A, Colin AA. Late preterm infants: near term but still in a critical developmental time period. Pediatrics. 2013; 132:741–51.5. McGowan JE, Alderdice FA, Holmes VA, Johnston L. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011; 127:1111–24.6. Heinonen K, Eriksson JG, Lahti J, Kajantie E, Pesonen AK, Tuovinen S, et al. Late preterm birth and neurocognitive performance in late adulthood: a birth cohort study. Pediatrics. 2015; 135:e818–25.7. Shah P, Kaciroti N, Richards B, Oh W, Lumeng JC. Developmental outcomes of late preterm infants from infancy to kindergarten. Pediatrics. 2016; 138:e20153496.8. Celik IH, Demirel G, Canpolat FE, Dilmen U. A common problem for neonatal intensive care units: late preterm infants, a prospective study with term controls in a large perinatal center. J Matern Fetal Neonatal Med. 2013; 26:459–62.9. Fleming PF, Arora P, Mitting R, Aladangady N. A national survey of admission practices for late preterm infants in England. BMC Pediatr. 2014; 14:150.10. Cuestas E, Gaido MI, Capra RH. Transient neonatal hyperthyrotropinemia is a risk factor for developing persistent hyperthyrotropinemia in childhood with repercussion on developmental status. Eur J Endocrinol. 2015; 172:483–90.11. Oppenheimer JH, Schwartz HL. Molecular basis of thyroid hormone-dependent brain development. Endocr Rev. 1997; 18:462–75.12. Grosse SD, Van Vliet G. Prevention of intellectual disability through screening for congenital hypothyroidism: how much and at what level? Arch Dis Child. 2011; 96:374–9.13. Van Wassenaer AG, Kok JH. Hypothyroxinaemia and thyroid function after preterm birth. Semin Neonatol. 2004; 9:3–11.14. American Academy of Pediatrics, Rose SR; Section on Endocrinology and Committee on Genetics; American Thyroid Association, Brown RS; Public Health Committee; Lawson Wilkins Pediatric Endocrine Society, Foley T, et al. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics. 2006; 117:2290–303.15. Mass Screening Committee; Japanese Society for Pediatric Endocrinology; Japanese Society for Mass Screening, Nagasaki K, Minamitani K, Anzo M, et al. Guidelines for mass screening of congenital hypothyroidism (2014 revision). Clin Pediatr Endocrinol. 2015; 24:107–33.16. Kucharska AM, Ben-Skowronek I, Walczak M, Oltarzewski M, Szalecki M, Jackowska T, et al. Congenital hypothyroidism: Polish recommendations for therapy, treatment monitoring, and screening tests in special categories of neonates with increased risk of hypothyroidism. Endokrynol Pol. 2016; 67:536–47.17. Leger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. Horm Res Paediatr. 2014; 81:80–103.18. Zung A, Yehieli A, Blau A, Almashanu S. Characteristics of delayed thyroid stimulating hormone elevation in neonatal intensive care unit newborns. J Pediatr. 2016; 178:135–40.19. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013; 13:59.20. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978; 92:529–34.21. Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet. 2006; 367:1421–31.22. Larson C, Hermos R, Delaney A, Daley D, Mitchell M. Risk factors associated with delayed thyrotropin elevations in congenital hypothyroidism. J Pediatr. 2003; 143:587–91.23. Woo HC, Lizarda A, Tucker R, Mitchell ML, Vohr B, Oh W, et al. Congenital hypothyroidism with a delayed thyroid-stimulating hormone elevation in very premature infants: incidence and growth and developmental outcomes. J Pediatr. 2011; 158:538–42.24. Sun X, Lemyre B, Nan X, Harrold J, Perkins SL, Lawrence SE, et al. Free thyroxine and thyroid-stimulating hormone reference intervals in very low birth weight infants at 3-6 weeks of life with the Beckman Coulter Unicel DxI 800. Clin Biochem. 2014; 47:16–8.25. Lee JH, Kim SW, Jeon GW, Sin JB. Thyroid dysfunction in very low birth weight preterm infants. Korean J Pediatr. 2015; 58:224–9.26. Kaluarachchi DC, Colaizy TT, Pesce LM, Tansey M, Klein JM. Congenital hypothyroidism with delayed thyroid-stimulating hormone elevation in premature infants born at less than 30 weeks gestation. J Perinatol. 2017; 37:277–82.27. Linder N, Davidovitch N, Reichman B, Kuint J, Lubin D, Meyerovitch J, et al. Topical iodine-containing antiseptics and subclinical hypothyroidism in preterm infants. J Pediatr. 1997; 131:434–9.28. Aitken J, Williams FL. A systematic review of thyroid dysfunction in preterm neonates exposed to topical iodine. Arch Dis Child Fetal Neonatal Ed. 2014; 99:F21–8.29. Ares S, Quero J, de Escobar GM. Iodine balance, iatrogenic excess, and thyroid dysfunction in premature newborns. Semin Perinatol. 2008; 32:407–12.30. Chung HR, Shin CH, Yang SW, Choi CW, Kim BI. Subclinical hypothyroidism in Korean preterm infants associated with high levels of iodine in breast milk. J Clin Endocrinol Metab. 2009; 94:4444–7.31. Bagnoli F, Farmeschi L, Peruzzi L, Musaraf A, Paffetti P, Badii S, et al. Altered thyroid function in small for gestational age newborns: study based on screening test for congenital hypothyroidism. J Pediatr Sci. 2010; 4:e55.32. Franco B, Laura F, Sara N, Salvatore G. Thyroid function in small for gestational age newborns: a review. J Clin Res Pediatr Endocrinol. 2013; 5 Suppl 1:2–7.33. Uchiyama A, Watanabe H, Nakanishi H, Totsu S. Small for gestational age is a risk factor for the development of delayed thyrotropin elevation in infants weighing less than 2000 g. Clin Endocrinol (Oxf). 2018; 89:431–6.34. Aktas ON, Gursoy T, Soysal E, Esencan E, Ercin S. Thyroid hormone levels in late preterm, early term and term infants: a study with healthy neonates revealing reference values and factors affecting thyroid hormones. J Pediatr Endocrinol Metab. 2017; 30:1191–6.35. Vigone MC, Caiulo S, Di Frenna M, Ghirardello S, Corbetta C, Mosca F, et al. Evolution of thyroid function in preterm infants detected by screening for congenital hypothyroidism. J Pediatr. 2014; 164:1296–302.36. Bijarnia S, Wilcken B, Wiley VC. Newborn screening for congenital hypothyroidism in very-low-birth-weight babies: the need for a second test. J Inherit Metab Dis. 2011; 34:827–33.37. Hashemipour M, Hovsepian S, Ansari A, Keikha M, Khalighinejad P, Niknam N. Screening of congenital hypothyroidism in preterm, low birth weight and very low birth weight neonates: a systematic review. Pediatr Neonatol. 2018; 59:3–14.38. Chung ML, Yoo HW, Kim KS, Lee BS, Pi SY, Lim G, et al. Thyroid dysfunctions of prematurity and their impacts on neurodevelopmental outcome. J Pediatr Endocrinol Metab. 2013; 26:449–55.39. Lim G, Lee YK, Han HS. Early discontinuation of thyroxine therapy is possible in most very low-birthweight infants with hypothyroidism detected by screening. Acta Paediatr. 2014; 103:e123. –9.40. Jung JM, Jin HY, Chung ML. Feasibility of an early discontinuation of thyroid hormone treatment in very-low-birth-weight infants at risk for transient or permanent congenital hypothyroidism. Horm Res Paediatr. 2016; 85:131–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparisons of Clinical Characteristics Affecting Readmission between Late Preterm Infants and Moderate Preterm Infants or Full-Term Infants
- Clinical Characteristics of Apnea in Full-Term Infants: Compared to Late Preterm Infants
- Assessment of Preterm Infants Using the Bayley-III Scales in Korea
- Effects of an Infant Care Education Program for Mothers of Late-preterm Infants on Parenting Confidence, Breastfeeding Rates, and Infants' Growth and Readmission Rates
- Neurodevelopmental Outcomes of Moderate-to-Late Preterm Infants