Korean J Physiol Pharmacol.
2020 Jan;24(1):19-26. 10.4196/kjpp.2020.24.1.19.
Medium- and long-chain triglyceride propofol reduces the activity of acetyl-coenzyme A carboxylase in hepatic lipid metabolism in HepG2 and Huh7 cells
- Affiliations
-
- 1Center for Anesthesiology, Beijing Anzhen Hospital, Capital Medical University, Beijing 100029, P.R China. majun7689@163.com
- 2North China University of Science and Technology, Tangshan, Hebei 063300, P.R China.
- KMID: 2466566
- DOI: http://doi.org/10.4196/kjpp.2020.24.1.19
Abstract
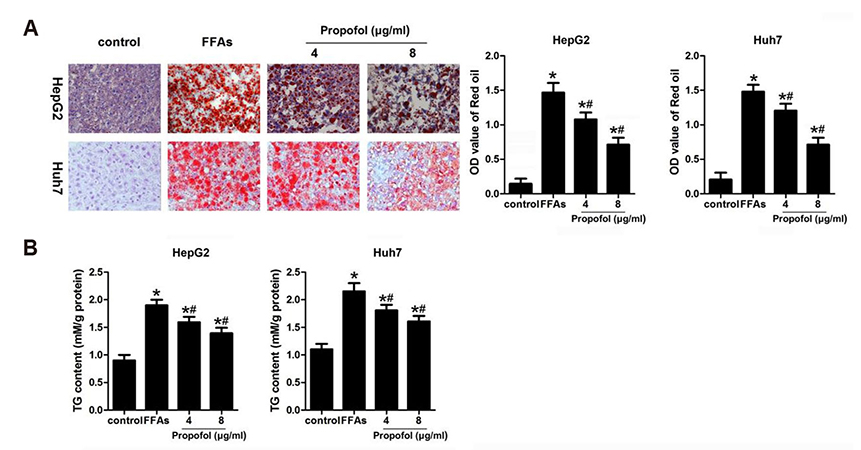
- Medium- and long-chain triglyceride (MCT/LCT) propofol is widely used as an intravenous anesthetic, especially in the intensive care unit. The present study aimed to assess whether MCT/LCT propofol is safe in the hyperlipidemic population for long-term use. Free fatty acids (FFAs) were used to establish high-fat stimulation of HepG2 and Huh7 cells. Subsequently, these cells were treated with propofol at the concentration of 0, 4, or 8 µg/ml for 24 and 48 h. The results indicated that the cell viability was notably decreased when the cells were stimulated with 2 mmol/L FFAs and treated with 12 µg/ml MCT/LCT propofol. Accordingly, we chose 2 mmol/L FFAs along with 4 and 8 µg/ml MCT/LCT propofol for the subsequent experiments. Four and 8 µg/ml MCT/LCT propofol inhibited FFA-induced lipid accumulation in the cells and significantly reversed acetyl coenzyme A carboxylase (ACC) activity. In addition, MCT/LCT propofol not only significantly promoted the phosphorylation of AMPK and ACC, but also reversed the FFA-induced decreased phosphorylation of AMPK and ACC. In conclusion, MCT/LCT propofol reverses the negative effects caused by FFAs in HepG2 and Huh7 cells, indicating that MCT/LCT propofol might positively regulate lipid metabolism.
Keyword
MeSH Terms
Figure
Reference
-
1. Erickson SK. Nonalcoholic fatty liver disease. J Lipid Res. 2009; 50 Suppl:S412–S416.
Article2. Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998; 114:842–845.
Article3. Singla B, Malde AD. A prospective observational study of injection pain in children with medium plus long chain triglyceride and long chain triglyceride propofol premixed with lignocaine. Indian J Anaesth. 2018; 62:214–218.
Article4. Yew WS, Chong SY, Tan KH, Goh MH. The effects of intravenous lidocaine on pain during injection of medium- and long-chain triglyceride propofol emulsions. Anesth Analg. 2005; 100:1693–1695.
Article5. Theilen HJ, Adam S, Albrecht MD, Ragaller M. Propofol in a medium-and long-chain triglyceride emulsion: pharmacological characteristics and potential beneficial effects. Anesth Analg. 2002; 95:923–929.6. Wu GJ, Lin YW, Tsai HC, Lee YW, Chen JT, Chen RM. Sepsis-induced liver dysfunction was ameliorated by propofol via suppressing hepatic lipid peroxidation, inflammation, and drug interactions. Life Sci. 2018; 213:279–286.
Article7. Tsuchiya H, Ueno T, Tanaka T, Matsuura N, Mizogami M. Comparative study on determination of antioxidant and membrane activities of propofol and its related compounds. Eur J Pharm Sci. 2010; 39:97–102.
Article8. Ge M, Yao W, Wang Y, Yuan D, Chi X, Luo G, Hei Z. Propofol alleviates liver oxidative stress via activating Nrf2 pathway. J Surg Res. 2015; 196:373–381.
Article9. Kobayashi K, Yoshino F, Takahashi SS, Todoki K, Maehata Y, Komatsu T, Yoshida K, Lee MC. Direct assessments of the antioxidant effects of propofol medium chain triglyceride/long chain triglyceride on the brain of stroke-prone spontaneously hypertensive rats using electron spin resonance spectroscopy. Anesthesiology. 2008; 109:426–435.
Article10. Musso G, Gambino R, Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog Lipid Res. 2009; 48:1–26.
Article11. Hastings IM, Hill WG. Analysis of lines of mice selected for fat content. 2. correlated responses in the activities of enzymes involved in lipogenesis. Genet Res. 1990; 55:55–61.
Article12. Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang D, Wang A, Zhang XM, Cline GW, Yu XX, Geisler JG, Bhanot S, Monia BP, Shulman GI. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006; 116:817–824.
Article13. Abu-Elheiga L, Matzuk MM, Kordari P, Oh W, Shaikenov T, Gu Z, Wakil SJ. Mutant mice lacking acetyl-CoA carboxylase 1 are embryonically lethal. Proc Natl Acad Sci U S A. 2005; 102:12011–12016.
Article14. Woo M, Song YO, Kang KH, Noh JS. Anti-obesity effects of collagen peptide derived from skate (raja kenojei) skin through regulation of lipid metabolism. Mar Drugs. 2018; 16:E306.
Article15. Yang X, Xu P, Zhang F, Zhang L, Zheng Y, Hu M, Wang L, Han TL, Peng C, Wang L, Wen L, Zeng Y, Gao R, Xia Y, Tong C, Yang Z, Qi H, Baker PN. AMPK Hyper-Activation Alters Fatty Acids Metabolism and Impairs Invasiveness of Trophoblasts in Preeclampsia. Cell Physiol Biochem. 2018; 49:578–594.
Article16. Ma J, Kang SY, Meng X, Kang AN, Park JH, Park YK, Jung HW. Effects of rhizome extract of dioscorea batatas and its active compound, allantoin, on the regulation of myoblast differentiation and mitochondrial biogenesis in c2c12 myotubes. Molecules. 2018; 23:E2023.
Article17. Huang Y, Hao J, Tian D, Wen Y, Zhao P, Chen H, Lv Y, Yang X. Antidiabetic Activity of a Flavonoid-Rich Extract From Sophora davidii (Franch.) Skeels in KK-Ay Mice via Activation of AMP-Activated Protein Kinase. Front Pharmacol. 2018; 9:760.
Article18. Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004; 3:340–351.
Article19. Dihingia A, Bordoloi J, Dutta P, Kalita J, Manna P. Hexane-isopropanolic extract of Tungrymbai, a North-East Indian fermented soybean food prevents hepatic steatosis via regulating AMPK-mediated SREBP/FAS/ACC/HMGCR and PPARα/CPT1A/UCP2 pathways. Sci Rep. 2018; 8:10021.
Article20. Jung S, Son H, Hwang CE, Cho KM, Park SW, Kim HJ. Ganoderma lucidum ameliorates non-alcoholic steatosis by upregulating energy metabolizing enzymes in the liver. J Clin Med. 2018; 7:E152.
Article21. Li RZ, Fan XX, Duan FG, Jiang ZB, Pan HD, Luo LX, Zhou YL, Li Y, Yao YJ, Yao XJ, Leung ELH, Liu L. Proscillaridin A induces apoptosis and suppresses non-small-cell lung cancer tumor growth via calcium-induced DR4 upregulation. Cell Death Dis. 2018; 9:696.
Article22. Hao J, Huang K, Chen C, Liang Y, Wang Y, Zhang X, Huang H. Polydatin improves glucose and lipid metabolisms in insulin-resistant HepG2 cells through the AMPK pathway. Biol Pharm Bull. 2018; 41:891–898.
Article23. Gong T, Ning X, Deng Z, Liu M, Zhou B, Chen X, Huang S, Xu Y, Chen Z, Luo R. Propofol-induced miR-219-5p inhibits growth and invasion of hepatocellular carcinoma through suppression of GPC3-mediated Wnt/β-catenin signalling activation. J Cell Biochem. 2019; 120:16934–16945.
Article24. Zhang J, Shan WF, Jin TT, Wu GQ, Xiong XX, Jin HY, Zhu SM. Propofol exerts anti-hepatocellular carcinoma by microvesicle-mediated transfer of miR-142-3p from macrophage to cancer cells. J Transl Med. 2014; 12:279.
Article25. Shirwany NA, Zou MH. AMPK: a cellular metabolic and redox sensor. A minireview. Front Biosci (Landmark Ed). 2014; 19:447–474.
Article26. Carling D, Hardie DG. The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim Biophys Acta. 1989; 1012:81–86.
Article27. Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005; 1:15–25.
Article28. Bijland S, Mancini SJ, Salt IP. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin Sci (Lond). 2013; 124:491–507.
Article29. Hardie DG. AMP-activated protein kinase: maintaining energy homeostasis at the cellular and whole-body levels. Annu Rev Nutr. 2014; 34:31–55.
Article30. Lin SC, Hardie DG. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab. 2018; 27:299–313.
Article31. Day EA, Ford RJ, Steinberg GR. AMPK as a Therapeutic Target for Treating Metabolic Diseases. Trends Endocrinol Metab. 2017; 28:545–560.
Article32. Liu H, Liu M, Jin Z, Yaqoob S, Zheng M, Cai D, Liu J, Guo S. Ginsenoside Rg2 inhibits adipogenesis in 3T3-L1 preadipocytes and suppresses obesity in high-fat-diet-induced obese mice through the AMPK pathway. Food Funct. 2019; 10:3603–3614.
Article33. Tsuji H, Kasai M, Takeuchi H, Nakamura M, Okazaki M, Kondo K. Dietary medium-chain triacylglycerols suppress accumulation of body fat in a double-blind, controlled trial in healthy men and women. J Nutr. 2001; 131:2853–2859.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of medicinal herb water extracts on expression of hepatic glucokinase, pyruvate dehydrogenase and acetyl-CoA carboxylase mRNA
- Regulation of acetyl CoA carboxylase mRNA in rat liver by high carbohydrate diet and insulin
- The effect of fucoxanthin rich power on the lipid metabolism in rats with a high fat diet
- Standardized rice bran extract improves hepatic steatosis in HepG2 cells and ovariectomized rats
- Nonalcoholic fatty liver disease: molecular mechanisms for the hepatic steatosis