Yonsei Med J.
2016 Jan;57(1):203-208. 10.3349/ymj.2016.57.1.203.
Development of a Synthetic Surfactant Using a Surfactant Protein-C Peptide Analog: In Vitro Studies of Surface Physical Properties
- Affiliations
-
- 1Department of Pediatrics, Kyung Hee University School of Medicine, Seoul, Korea. baecw@khnmc.or.kr
- KMID: 2466371
- DOI: http://doi.org/10.3349/ymj.2016.57.1.203
Abstract
- PURPOSE
Pulmonary surfactant (PS) replacement has been the gold standard therapy for neonatal respiratory distress syndrome; however, almost all commercial PSs contain animal proteins. We prepared a synthetic PS by using a human surfactant protein (SP) analog and evaluated its in vitro properties.
MATERIALS AND METHODS
A peptide sequence (CPVHLKRLLLLLLLLLLLLLLLL) of human SP-C was chosen to develop the peptide analog (SPa-C). The new synthetic SP-C PS (sSP-C PS) was synthesized from SPa-C, dipalmitoyl phosphatidylcholine, phosphatidyl glycerol, and palmitic acid. Physical properties of the sSP-C PS were evaluated by measuring the maximum and minimum surface tensions (STs), surfactant spreading, and adsorption rate. In addition, we recorded an ST-area diagram. The data obtained on sSP-C PS were subsequently compared with those of purified natural bovine surfactant (PNBS), and the commercial product, Surfacten(R).
RESULTS
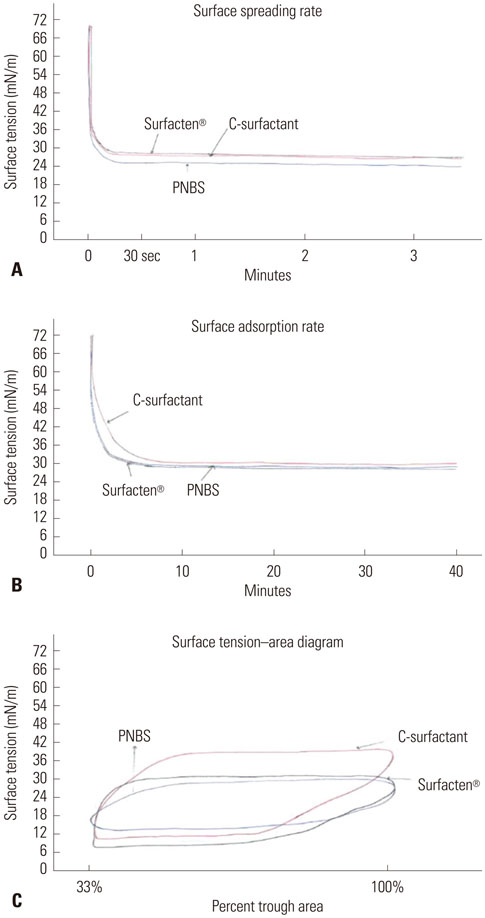
The sSP-C PS and Surfacten(R) were found to have maximum ST values of 32-33 mN/m, whereas that of PNBS was much lower at 19 mN/m. The minimum ST values of all three products were less than 10 mN/m. The values that were measured for the equilibrium ST of rapidly spreading sSP-C PS, Surfacten(R), and PNBS were 27, 27, and 24 mN/m, respectively. The surface adsorptions were found to be the same for all three PSs (20 mN/m). ST-area diagrams of sSP-C PS and Surfacten(R) revealed similar properties.
CONCLUSION
In an in vitro experiment, the physical properties exhibited by sSP-C PS were similar to those of Surfacten(R). Further study is required to evaluate the in vivo efficacy.
Keyword
MeSH Terms
-
1,2-Dipalmitoylphosphatidylcholine/analogs & derivatives
Adsorption
Amino Acid Sequence/*genetics
Animals
C-Peptide/*chemistry
Cattle
Humans
Infant, Newborn
Pulmonary Surfactant-Associated Protein C/*chemical synthesis/pharmacology
Pulmonary Surfactants/*chemical synthesis/pharmacology
Respiratory Distress Syndrome, Newborn/*drug therapy
*Surface Properties
*Surface Tension
Surface-Active Agents
C-Peptide
1,2-Dipalmitoylphosphatidylcholine
Pulmonary Surfactant-Associated Protein C
Pulmonary Surfactants
Surface-Active Agents
Figure
Cited by 2 articles
-
History of Pulmonary Surfactant Replacement Therapy for Neonatal Respiratory Distress Syndrome in Korea
Chong-Woo Bae, Chae Young Kim, Sung-Hoon Chung, Yong-Sung Choi
J Korean Med Sci. 2019;34(25):. doi: 10.3346/jkms.2019.34.e175.A Combination of Short and Simple Surfactant Protein B and C Analogues as a New Synthetic Surfactant:
In Vitro and Animal Experiments
Yong-Sung Choi, Sung-Hoon Chung, Chong-Woo Bae
Yonsei Med J. 2017;58(4):823-828. doi: 10.3349/ymj.2017.58.4.823.
Reference
-
1. Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants--2013 update. Neonatology. 2013; 103:353–368.
Article2. Soll R, Ozek E. Multiple versus single doses of exogenous surfactant for the prevention or treatment of neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2009; (1):CD000141.
Article3. Halliday HL. Surfactants: past, present and future. J Perinatol. 2008; 28:Suppl 1. S47–S56.
Article4. Bae CW, Kwon YD, Ko SJ, Kim KS, Kim HM, Park WS, et al. Surfactant replacement therapy in neonates with respiratory distress syndrome: a collective evaluation of trials from 16 hospitals. J Korean Pediatr Soc. 1993; 36:244–265.5. Bae CW. Surfactant replacement therapy in RDS: a collaborative study of multi-center trials in Korea. J Korean Soc Neonatol. 1997; 4:124–135.6. Bae CW, Kim YM. Surfactant therapy for neonatal respiratory distress syndrome: experience in Korea over 15 years. Korean J Pediatr. 2004; 47:940–948.7. Bae CW, Hahn WH. Surfactant therapy for neonatal respiratory distress syndrome: a review of Korean experiences over 17 years. J Korean Med Sci. 2009; 24:1110–1118.
Article8. Bae CW, Hahn WH, Chang JY, Kim SM. Surfactant replacement therapy for RDS: a collaborative study of 72 multi-center trials in Korea (2010) and a review of Korean experiences over 20 years. J Korean Soc Neonatol. 2011; 18:409–411.
Article9. Kim SM, Park YJ, Chung SH, Choi YS, Kim CH, Bae CW. Early prophylactic versus late selective use of surfactant for respiratory distress syndrome in very preterm infants: a collaborative study of 53 multi-center trials in Korea. J Korean Med Sci. 2014; 29:1126–1131.
Article10. Moya FR, Gadzinowski J, Bancalari E, Salinas V, Kopelman B, Bancalari A, et al. A multicenter, randomized, masked, comparison trial of lucinactant, colfosceril palmitate, and beractant for the prevention of respiratory distress syndrome among very preterm infants. Pediatrics. 2005; 115:1018–1029.
Article11. Davis AJ, Jobe AH, Häfner D, Ikegami M. Lung function in premature lambs and rabbits treated with a recombinant SP-C surfactant. Am J Respir Crit Care Med. 1998; 157:553–559.
Article12. Pfister RH, Soll RF. New synthetic surfactants: the next generation? Biol Neonate. 2005; 87:338–344.
Article13. Almlén A, Walther FJ, Waring AJ, Robertson B, Johansson J, Curstedt T. Synthetic surfactant based on analogues of SP-B and SP-C is superior to single-peptide surfactants in ventilated premature rabbits. Neonatology. 2010; 98:91–99.
Article14. Seehase M, Collins JJ, Kuypers E, Jellema RK, Ophelders DR, Ospina OL, et al. New surfactant with SP-B and C analogs gives survival benefit after inactivation in preterm lambs. PLoS One. 2012; 7:e47631.
Article15. Otsubo E, Irimajiri K, Takei T, Nomura M. Characterization of synthetic lung surfactant activity against proinflammatory cytokines in human monocytes. Biol Pharm Bull. 2002; 25:312–317.
Article16. Bae CW, Ahn CI, Kim KL, Hahm KS, Takahashi A, Fujiwara T. Purification of bovine lung natural surfactant and assessment of surface physical properties. J Korean Med Assoc. 1991; 34:534–544.17. Fujiwara T, Konishi M, Chida S, Okuyama K, Ogawa Y, Takeuchi Y, et al. Surfactant replacement therapy with a single postventilatory dose of a reconstituted bovine surfactant in preterm neonates with respiratory distress syndrome: final analysis of a multicenter, double-blind, randomized trial and comparison with similar trials. The surfactant-TA study group. Pediatrics. 1990; 86:753–764.
Article18. Takei T, Hashimoto Y, Aiba T, Sakai K, Fujiwara T. The surface properties of chemically synthesized peptides analogous to human pulmonary surfactant protein SP-C. Biol Pharm Bull. 1996; 19:1247–1253.
Article19. Kang JH, Shin SY, Maeng CY, Kim KL, Bae CW, Hahm KS. Preparation and in vitro physical activities of crude natural surfactant and artificial pulmonary surfactant containing synthetic peptide and phospholipid mixtures. J Korean Pediatr Soc. 1998; 41:472–480.20. Shelley SA, Paciga JE, Balis JU. Purification of surfactant from lung washings and washings contaminated with blood constituents. Lipids. 1977; 12:505–510.
Article21. Fujiwara T, Robertson B. Pharmacology of exogenous surfactant. In : Robertson B, Van Golde LMG, Batenburg JJ, editors. Pulmonary surfactant: from molecular biology to clinical practice. 2nd ed. Amsterdam: Elservier;1992. p. 561–592.22. Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants - 2010 update. Neonatology. 2010; 97:402–417.
Article23. Ma CC, Ma S. The role of surfactant in respiratory distress syndrome. Open Respir Med J. 2012; 6:44–53.
Article24. Ramanathan R. Animal-derived surfactants: where are we? The evidence from randomized, controlled clinical trials. J Perinatol. 2009; 29:Suppl 2. S38–S43.
Article25. Soll RF, Blanco F. Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2001; (2):CD000144.
Article26. Kirsten GF, Kirsten CL, Henning PA, Smith J, Holgate SL, Bekker A, et al. The outcome of ELBW infants treated with NCPAP and In-SurE in a resource-limited institution. Pediatrics. 2012; 129:e952–e959.
Article27. Jeon GW, Oh M, Sin JB. Efficacy of surfactant-TA, calfactant and poractant alfa for preterm infants with respiratory distress syndrome: a retrospective study. Yonsei Med J. 2015; 56:433–439.
Article28. Seger N, Soll R. Animal derived surfactant extract for treatment of respiratory distress syndrome. Cochrane Database Syst Rev. 2009; (2):CD007836.
Article29. Soll RF, Morley CJ. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2001; (2):CD000510.
Article30. Bahadue FL, Soll R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2012; 11:CD001456.
Article31. Stevens TP, Harrington EW, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev. 2007; (4):CD003063.
Article32. Kim YD. New synthetic surfactants for neonates. J Korean Soc Neonatol. 2012; 19:184–194.
Article33. Curstedt T, Johansson J. New synthetic surfactants--basic science. Biol Neonate. 2005; 87:332–337.34. Spragg RG, Lewis JF, Wurst W, Häfner D, Baughman RP, Wewers MD, et al. Treatment of acute respiratory distress syndrome with recombinant surfactant protein C surfactant. Am J Respir Crit Care Med. 2003; 167:1562–1566.
Article35. Spragg RG, Lewis JF, Walmrath HD, Johannigman J, Bellingan G, Laterre PF, et al. Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med. 2004; 351:884–892.
Article36. Curstedt T. Surfactant protein C: basics to bedside. J Perinatol. 2005; 25:Suppl 2. S36–S38.
Article37. Johansson J, Nilsson G, Strömberg R, Robertson B, Jörnvall H, Curstedt T. Secondary structure and biophysical activity of synthetic analogues of the pulmonary surfactant polypeptide SP-C. Biochem J. 1995; 307(Pt 2):535–541.
Article38. Nilsson G, Gustafsson M, Vandenbussche G, Veldhuizen E, Griffiths WJ, Sjövall J, et al. Synthetic peptide-containing surfactants--evaluation of transmembrane versus amphipathic helices and surfactant protein C poly-valyl to poly-leucyl substitution. Eur J Biochem. 1998; 255:116–124.
Article39. Takei T, Hashimoto Y, Ohtsubo E, Sakai K, Ohkawa H. Characterization of poly-leucine substituted analogues of the human surfactant protein SP-C. Biol Pharm Bull. 1996; 19:1550–1555.
Article40. Otsubo E, Takei T. Effects of the human pulmonary surfactant protein-C (SP-C), SP-CL16(6-28) on surface activities of surfactants with various phospholipids. Biol Pharm Bull. 2002; 25:1303–1306.
Article41. Fujiwara T, Maeta H, Chida S, Morita T, Watabe Y, Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980; 1:55–59.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum to “Development of a Synthetic Surfactant Using a Surfactant Protein-C Peptide Analog: In Vitro Studies of Surface Physical Properties†by Bae CW, et al. (Yonsei Med J 2016;57(1):203–208.)
- Preparation and in vitro Physical Activities of Crude Natural Surfactant and Artificial Pulmonary Surfactant Containing Synthetic Peptide and Phospholipid Mixtures
- The use of artificial pulmonary surfactant in neonatal respiratory distress
- A Study of Surface Physical Properties of New Surfactant Using Synthetic Peptides of Surfactant Protein-B
- In vitro effect of meconium on the physical surface properties and morphology of exogenous pulmonary surfactant