J Pathol Transl Med.
2019 Mar;53(2):75-85. 10.4132/jptm.2018.10.11.
Loss of Human Leukocyte Antigen Class I Expression Is Associated with Poor Prognosis in Patients with Advanced Breast Cancer
- Affiliations
-
- 1Department of Hospital Pathology, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. chj0103@catholic.ac.kr
- 2Department of Surgery, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2465446
- DOI: http://doi.org/10.4132/jptm.2018.10.11
Abstract
- BACKGROUND
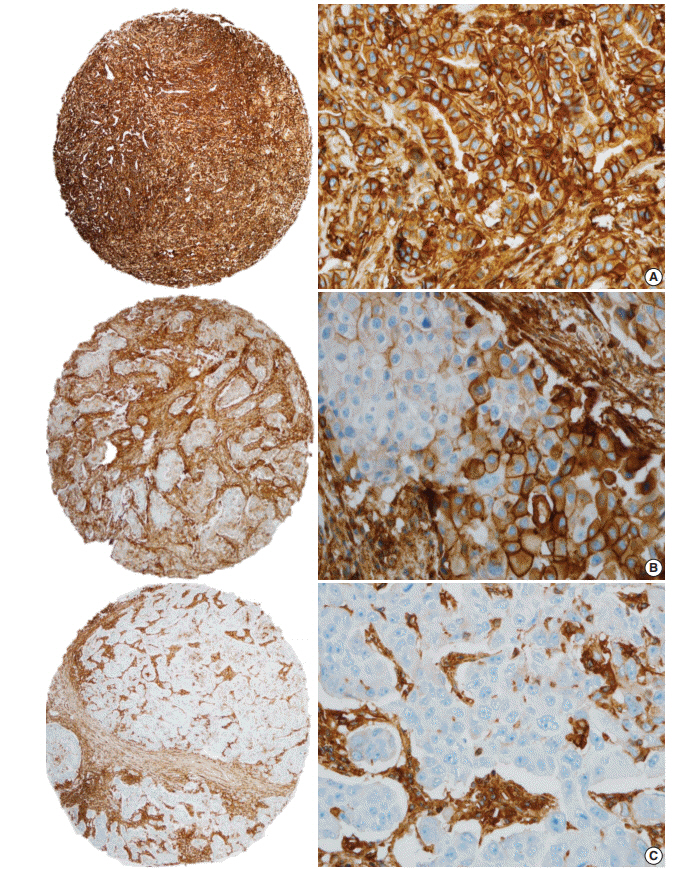
Human leukocyte antigen class I (HLA-I) molecules play important roles in regulating immune responses. Loss or reduction of HLA-I expression has been shown to be associated with prognosis in several cancers. Regulatory T-cells (Tregs) also play critical functions in immune response regulation. Evaluation of HLA-I expression status by the EMR8-5 antibody and its clinical impact in breast cancer have not been well studied, and its relationship with Tregs remains unclear.
METHODS
We evaluated HLA-I expression and Treg infiltration by immunohistochemistry in 465 surgically resected breast cancer samples. We examined the correlation between HLA-I expression and Treg infiltration and clinicopathologic characteristics and survival analyses were performed.
RESULTS
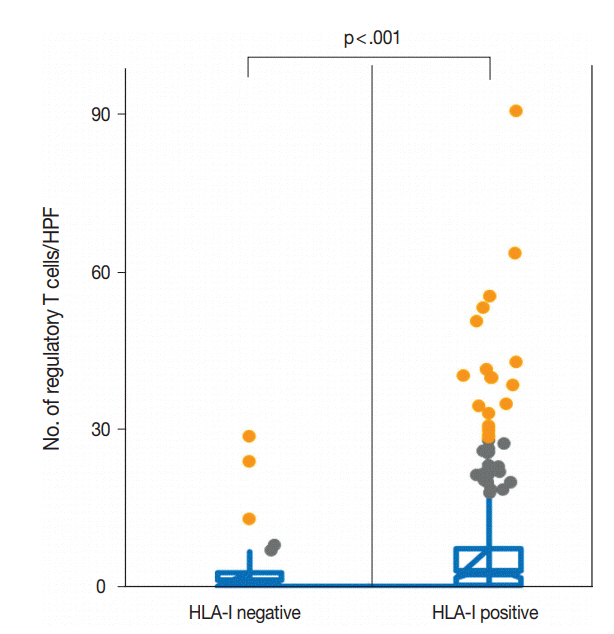
Total loss of HLA-I expression was found in 84 breast cancer samples (18.1%). Univariate survival analysis revealed that loss of HLA-I expression was significantly associated with worse disease-specific survival (DSS) (p = .029). HLA-I was not an independent prognostic factor in the entire patient group, but it was an adverse independent prognostic factor for DSS in patients with advanced disease (stage II-IV) (p = .031). Treg numbers were significantly higher in the intratumoral stroma of HLA-I-positive tumors than in HLA-I-negative tumors (median 6.3 cells/high power field vs 2.1 cells/high power field, p < .001). However, Tregs were not an independent prognostic factor in our cohort.
CONCLUSIONS
Our findings suggest that the loss of HLA-I expression is associated with poor prognosis in breast cancer patients, highlighting the role of HLA-I alterations in immune evasion mechanisms of breast cancer. HLA-I could be a promising marker that enables the application of more effective and precise immunotherapies for patients with advanced breast cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144:646–74.
Article2. Aptsiauri N, Cabrera T, Garcia-Lora A, Lopez-Nevot MA, Ruiz-Cabello F, Garrido F. MHC class I antigens and immune surveillance in transformed cells. Int Rev Cytol. 2007; 256:139–89.
Article3. Hicklin DJ, Marincola FM, Ferrone S. HLA class I antigen downregulation in human cancers: T-cell immunotherapy revives an old story. Mol Med Today. 1999; 5:178–86.
Article4. Garrido F, Ruiz-Cabello F, Cabrera T, et al. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today. 1997; 18:89–95.
Article5. Madjd Z, Spendlove I, Pinder SE, Ellis IO, Durrant LG. Total loss of MHC class I is an independent indicator of good prognosis in breast cancer. Int J Cancer. 2005; 117:248–55.
Article6. Torigoe T, Asanuma H, Nakazawa E, et al. Establishment of a monoclonal anti-pan HLA class I antibody suitable for immunostaining of formalin-fixed tissue: unusually high frequency of down-regulation in breast cancer tissues. Pathol Int. 2012; 62:303–8.
Article7. Chang CC, Campoli M, Restifo NP, Wang X, Ferrone S. Immune selection of hot-spot beta 2-microglobulin gene mutations, HLA-A2 allospecificity loss, and antigen-processing machinery component down-regulation in melanoma cells derived from recurrent metastases following immunotherapy. J Immunol. 2005; 174:1462–71.8. de Kruijf EM, van Nes JG, Sajet A, et al. The predictive value of HLA class I tumor cell expression and presence of intratumoral Tregs for chemotherapy in patients with early breast cancer. Clin Cancer Res. 2010; 16:1272–80.
Article9. Lee HJ, Song IH, Park IA, et al. Differential expression of major histocompatibility complex class I in subtypes of breast cancer is associated with estrogen receptor and interferon signaling. Oncotarget. 2016; 7:30119–32.
Article10. Kaneko K, Ishigami S, Kijima Y, et al. Clinical implication of HLA class I expression in breast cancer. BMC Cancer. 2011; 11:454.
Article11. Lee HJ, Kim JY, Park IA, et al. Prognostic significance of tumor-infiltrating lymphocytes and the tertiary lymphoid structures in HER2-positive breast cancer treated with adjuvant trastuzumab. Am J Clin Pathol. 2015; 144:278–88.
Article12. Chung YR, Kim HJ, Jang MH, Park SY. Prognostic value of tumor infiltrating lymphocyte subsets in breast cancer depends on hormone receptor status. Breast Cancer Res Treat. 2017; 161:409–20.
Article13. Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015; 35 Suppl:S185–98.
Article14. Ishigami S, Arigami T, Uenosono Y, et al. Cancerous HLA class I expression and regulatory T cell infiltration in gastric cancer. Cancer Immunol Immunother. 2012; 61:1663–9.
Article15. Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004; 10:942–9.
Article16. deLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012; 18:3022–9.
Article17. Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006; 24:5373–80.
Article18. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer;2010.19. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998; 11:155–68.20. Liu Y, Komohara Y, Domenick N, et al. Expression of antigen processing and presenting molecules in brain metastasis of breast cancer. Cancer Immunol Immunother. 2012; 61:789–801.
Article21. Tsukahara T, Kawaguchi S, Torigoe T, et al. Prognostic significance of HLA class I expression in osteosarcoma defined by anti-pan HLA class I monoclonal antibody, EMR8-5. Cancer Sci. 2006; 97:1374–80.
Article22. Gettinger S, Choi J, Hastings K, et al. Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov. 2017; 7:1420–35.
Article23. Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016; 375:819–29.
Article24. Del Campo AB, Carretero J, Muñoz JA, et al. Adenovirus expressing beta2-microglobulin recovers HLA class I expression and antitumor immunity by increasing T-cell recognition. Cancer Gene Ther. 2014; 21:317–32.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human Leukocyte Antigen Class I and Programmed Death-Ligand 1 Coexpression Is an Independent Poor Prognostic Factor in Adenocarcinoma of the Lung
- Significance of PCNA Oncoprotein as a Prognostic Predictor for Human Breast Cancer
- Prognostic Impact and Clinicopathological Correlation of CD133 and ALDH1 Expression in Invasive Breast Cancer
- alphaB-Crystallin is a Novel Oncoprotein Associated with Poor Prognosis in Breast Cancer
- FAS (Fatty acid synthase) expression in breast cancer