J Korean Soc Radiol.
2019 Nov;80(6):1203-1213. 10.3348/jksr.2019.80.6.1203.
MRI Criteria for Predicting Invasive Lesions in Biopsy-Proven Ductal Carcinoma in Situ
- Affiliations
-
- 1Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. claudel@skku.edu
- 2Department of Radiology, Ewha Womans University Seoul Hospital, Seoul, Korea.
- 3Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2464917
- DOI: http://doi.org/10.3348/jksr.2019.80.6.1203
Abstract
- PURPOSE
To evaluate the criteria for predicting invasive lesions with preoperative breast MRI in ductal carcinoma in situ (DCIS) histopathologically diagnosed with biopsy.
MATERIALS AND METHODS
We retrospectively analyzed the preoperative MRI findings of 80 percutaneous biopsy-proven DCIS. The morphological type, enhancement distribution and kinetics, and extent of the lesions were analyzed. We compared the results of pure DCIS and DCIS with invasive lesions. We evaluated the MRI criteria for predicting DCIS with invasive lesions and assessed its diagnostic performance.
RESULTS
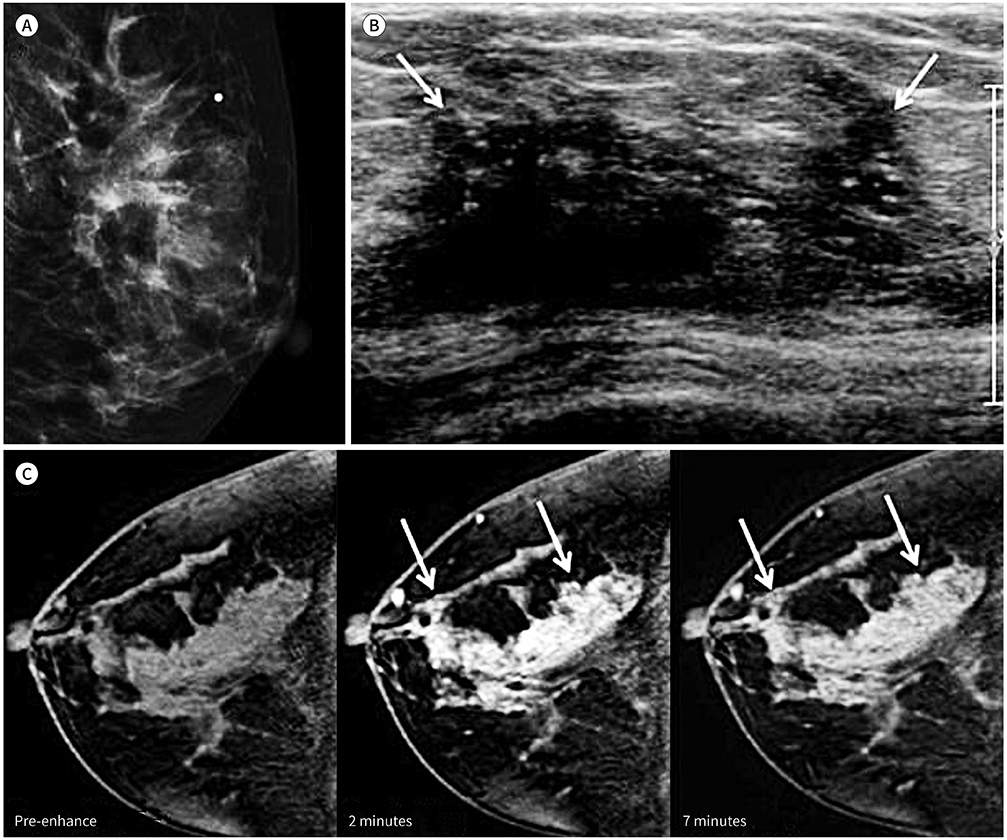
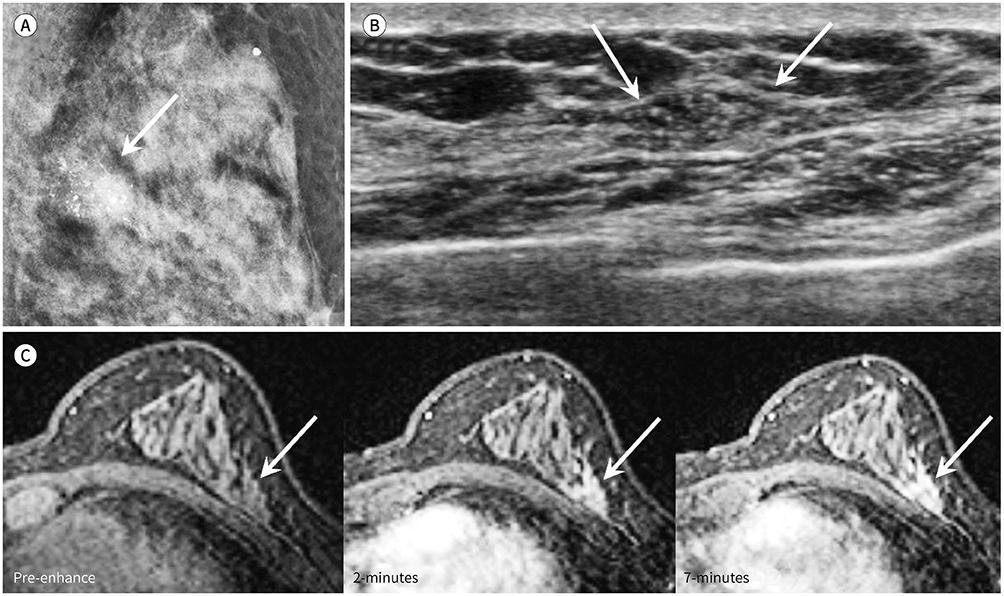
Of the 80 DCIS lesions analyzed, 27 contained co-existing invasive lesions and 49 were pure DCIS. No residual lesions after biopsy were seen in 4 cases. DCIS with invasive lesions showed washout kinetics more frequently and to a larger extent than did pure DCIS (p = 0.030 and p = 0.048, respectively). Using enhancement kinetics and the lesion cut-off value of 4 cm yielded the highest diagnostic performance, with 92.6% sensitivity and 93.8% negative predictive value for predicting invasive lesions.
CONCLUSION
Washout kinetics and the lesion extent of at least 4 cm are useful criteria for the prediction of co-existing invasive lesions in patients with DCIS diagnosed with biopsy.
MeSH Terms
Figure
Reference
-
1. Leonard GD, Swain SM. Ductal carcinoma in situ, complexities and challenges. J Natl Cancer Inst. 2004; 96:906–920.2. Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010; 102:170–178.3. Brennan ME, Turner RM, Ciatto S, Marinovich ML, French JR, Macaskill P, et al. Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology. 2011; 260:119–128.4. Jackman RJ, Burbank F, Parker SH, Evans WP 3rd, Lechner MC, Richardson TR, et al. Stereotactic breast biopsy of nonpalpable lesions: determinants of ductal carcinoma in situ underestimation rates. Radiology. 2001; 218:497–502.5. Lomoschitz FM, Helbich TH, Rudas M, Pfarl G, Linnau KF, Stadler A, et al. Stereotactic 11-gauge vacuum-assisted breast biopsy: influence of number of specimens on diagnostic accuracy. Radiology. 2004; 232:897–903.6. Pandelidis S, Heiland D, Jones D, Stough K, Trapeni J, Suliman Y. Accuracy of 11-gauge vacuum-assisted core biopsy of mammographic breast lesions. Ann Surg Oncol. 2003; 10:43–47.7. Intra M, Rotmensz N, Veronesi P, Colleoni M, Iodice S, Paganelli G, et al. Sentinel node biopsy is not a standard procedure in ductal carcinoma in situ of the breast: the experience of the European institute of oncology on 854 patients in 10 years. Ann Surg. 2008; 247:315–319.8. Patani N, Cutuli B, Mokbel K. Current management of DCIS: a review. Breast Cancer Res Treat. 2008; 111:1–10.9. Sakorafas GH, Farley DR, Peros G. Recent advances and current controversies in the management of DCIS of the breast. Cancer Treat Rev. 2008; 34:483–497.10. Yi M, Krishnamurthy S, Kuerer HM, Meric-Bernstam F, Bedrosian I, Ross MI, et al. Role of primary tumor characteristics in predicting positive sentinel lymph nodes in patients with ductal carcinoma in situ or microinvasive breast cancer. Am J Surg. 2008; 196:81–87.11. Berg WA, Gutierrez L, NessAiver MS, Carter WB, Bhargavan M, Lewis RS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004; 233:830–849.12. Medeiros LR, Duarte CS, Rosa DD, Edelweiss MI, Edelweiss M, Silva FR, et al. Accuracy of magnetic resonance in suspicious breast lesions: a systematic quantitative review and meta-analysis. Breast Cancer Res Treat. 2011; 126:273–285.13. Kuhl C, Kuhn W, Braun M, Schild H. Pre-operative staging of breast cancer with breast MRI: one step forward, two steps back? Breast. 2007; 16:Suppl 2. S34–S44.14. Esserman LJ, Kumar AS, Herrera AF, Leung J, Au A, Chen YY, et al. Magnetic resonance imaging captures the biology of ductal carcinoma in situ. J Clin Oncol. 2006; 24:4603–4610.15. Tardivon AA, Athanasiou A, Thibault F, El Khoury C. Breast imaging and reporting data system (BIRADS): magnetic resonance imaging. Eur J Radiol. 2007; 61:212–215.16. Hwang ES, Kinkel K, Esserman LJ, Lu Y, Weidner N, Hylton NM. Magnetic resonance imaging in patients diagnosed with ductal carcinoma-in-situ: value in the diagnosis of residual disease, occult invasion, and multicentricity. Ann Surg Oncol. 2003; 10:381–388.17. Morakkabati-Spitz N, Leutner C, Schild H, Traeber F, Kuhl C. Diagnostic usefulness of segmental and linear enhancement in dynamic breast MRI. Eur Radiol. 2005; 15:2010–2017.18. Guidi AJ, Fischer L, Harris JR, Schnitt SJ. Microvessel density and distribution in ductal carcinoma in situ of the breast. J Natl Cancer Inst. 1994; 86:614–619.19. Oshida K, Nagashima T, Ueda T, Yagata H, Tanabe N, Nakano S, et al. Pharmacokinetic analysis of ductal carcinoma in situ of the breast using dynamic MR mammography. Eur Radiol. 2005; 15:1353–1360.20. Stomper PC, Winston JS, Herman S, Klippenstein DL, Arredondo MA, Blumenson LE. Angiogenesis and dynamic MR imaging gadolinium enhancement of malignant and benign breast lesions. Breast Cancer Res Treat. 1997; 45:39–46.21. Van Goethem M, Schelfout K, Kersschot E, Colpaert C, Weyler J, Verslegers I, et al. Comparison of MRI features of different grades of DCIS and invasive carcinoma of the breast. JBR-BTR. 2005; 88:225–232.22. Trentin C, Dominelli V, Maisonneuve P, Menna S, Bazolli B, Luini A, et al. Predictors of invasive breast cancer and lymph node involvement in ductal carcinoma in situ initially diagnosed by vacuum-assisted breast biopsy: experience of 733 cases. Breast. 2012; 21:635–640.23. Yen TW, Hunt KK, Ross MI, Mirza NQ, Babiera GV, Meric-Bernstam F, et al. Predictors of invasive breast cancer in patients with an initial diagnosis of ductal carcinoma in situ: a guide to selective use of sentinel lymph node biopsy in management of ductal carcinoma in situ. J Am Coll Surg. 2005; 200:516–526.24. Lee JW, Han W, Ko E, Cho J, Kim EK, Jung SY, et al. Sonographic lesion size of ductal carcinoma in situ as a preoperative predictor for the presence of an invasive focus. J Surg Oncol. 2008; 98:15–20.25. Lee JM, Kaplan JB, Murray MP, Mazur-Grbec M, Tadic T, Stimac D, et al. Underestimation of DCIS at MRI-guided vacuum-assisted breast biopsy. AJR Am J Roentgenol. 2007; 189:468–474.26. Silverstein MJ, Waisman JR, Gamagami P, Gierson ED, Colburn WJ, Rosser RJ, et al. Intraductal carcinoma of the breast (208 cases). Clinical factors influencing treatment choice. Cancer. 1990; 66:102–108.27. Tweedie E, Tonkin K, Kerkvliet N, Doig GS, Sparrow RK, O'Malley FP. Biologic characteristics of breast cancer detected by mammography and by palpation in a screening program: a pilot study. Clin Invest Med. 1997; 20:300–307.28. Go EM, Chan SK, Vong JS, Lui PC, Chan AW, Ma TK, et al. Predictors of invasion in needle core biopsies of the breast with ductal carcinoma in situ. Mod Pathol. 2010; 23:737–742.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Unusual Presentation of Extensive Ductal Carcinoma in Situ Accompanying Invasive Ductal Carcinoma on MRI: A Case Report
- Invasive Lobular Carcinoma of the Breast Associated with Mixed Lobular and Ductal Carcinoma In Situ: A Case Report
- Predictive Factors of Residual Invasive Breast Cancer after Core Biopsy for Ductal Carcinoma in Situ
- A Method to Quantify Breast MRI for Predicting Tumor Invasion in Patients with Preoperative Biopsy- Proven Ductal Carcinoma in Situ (DCIS)
- MR Findings of Papillary Neoplasms of the Breast