Korean J Gastroenterol.
2019 Nov;74(5):274-280. 10.4166/kjg.2019.74.5.274.
Brief Review of the Revised Korean Association for the Study of the Liver Clinical Practice Guidelines for Liver Cirrhosis: Varices, Hepatic Encephalopathy and Related Complications
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. janges@snubh.org
- KMID: 2464186
- DOI: http://doi.org/10.4166/kjg.2019.74.5.274
Abstract
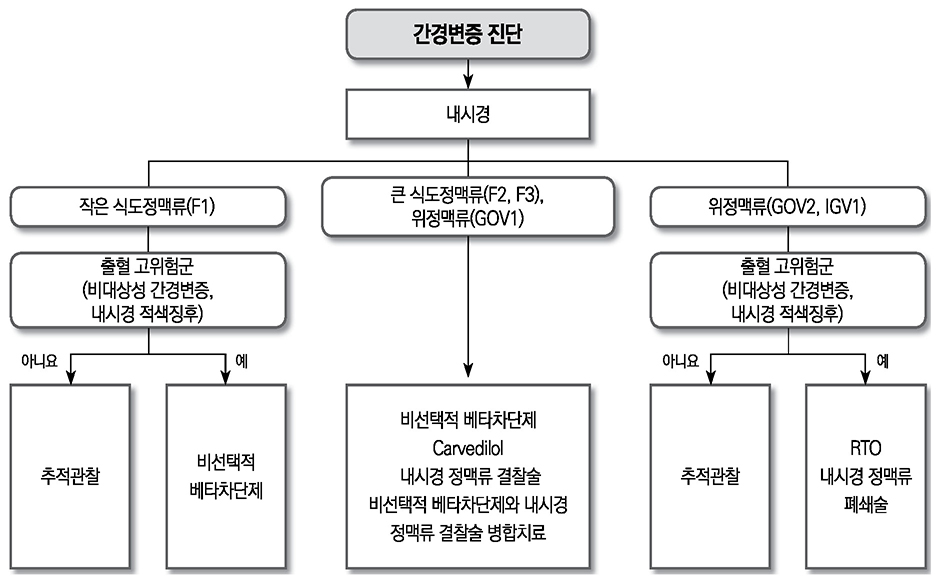
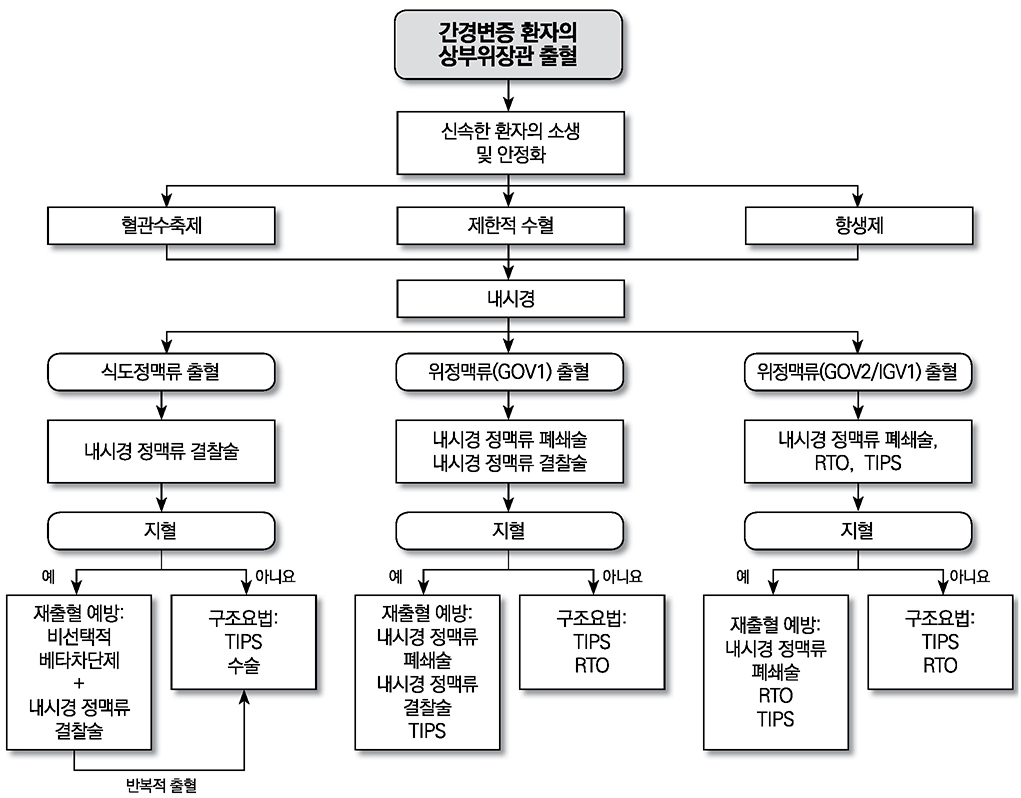
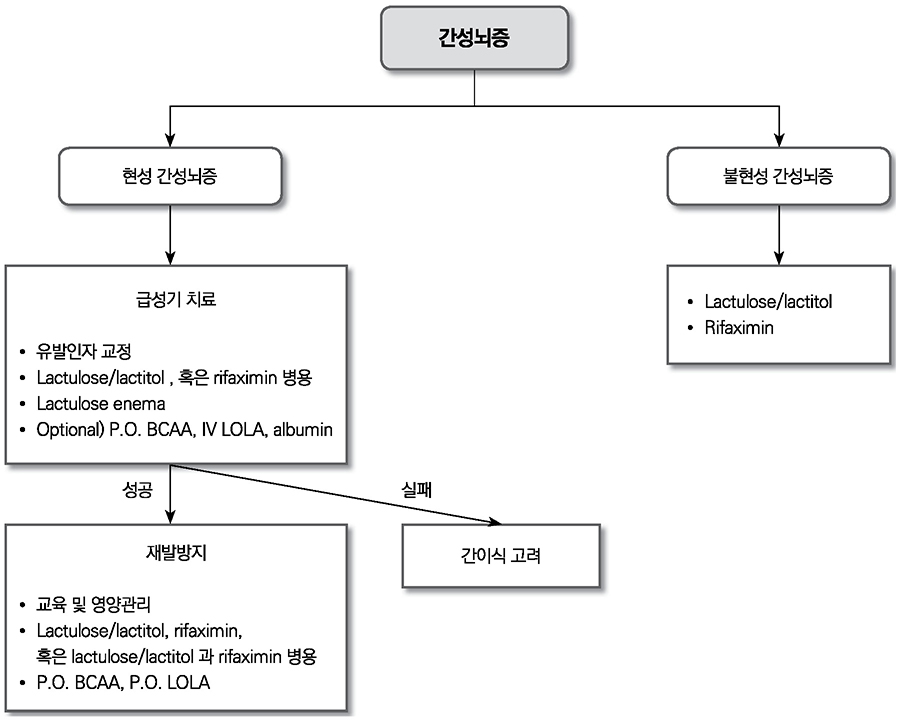
- Liver cirrhosis patients are suffering from many complications, which are directly related to a poor prognosis. Although there have been many recent advances in diagnosis and treatment for varix and hepatic encephalopathy in cirrhotic patients, the standard practice for these conditions should consider the different medical resources and etiology of these liver diseases among various countries. The Korean Association for the Study of the Liver published in 2005 a clinical practice guideline for the treatment of cirrhosis complications, and this year, they revised the guideline for treating gastroesophageal varices and hepatic encephalopathy. This review summarizes the revised practice guideline and emphasizes the updated recommendation.
MeSH Terms
Figure
Reference
-
1. Cause-of-death statistics in 2018 in the Republic of Korea. [Internet]. Daejeon: Statistics Korea;2019. 09. 24. cited 2019 Oct 13. Available from: http://kostat.go.kr/portal/korea/kor_nw/1/6/2/index.board?bmode=read&bSeq=&aSeq=377606&pageNo=1&rowNum=10&navCount=10&currPg=&searchInfo=&sTarget=title&sTxt=.2. Korean Association for the Study of the Liver. 2019 Korean Association for the Study of the Liver clinical practice guidelines for liver cirrhosis: varices, hepatic encephalopathy and related complications. Seoul: Jin Publishing Co.;2019.3. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008; 336:924–926.4. de Franchis R. Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015; 63:743–752.5. Merli M, Nicolini G, Angeloni S, et al. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol. 2003; 38:266–272.6. Groszmann RJ, Garcia-Tsao G, Bosch J, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005; 353:2254–2261.7. Sarin SK, Mishra SR, Sharma P, Sharma BC, Kumar A. Early primary prophylaxis with beta-blockers does not prevent the growth of small esophageal varices in cirrhosis: a randomized controlled trial. Hepatol Int. 2013; 7:248–256.8. Qi XS, Bao YX, Bai M, Xu WD, Dai JN, Guo XZ. Nonselective beta-blockers in cirrhotic patients with no or small varices: a meta-analysis. World J Gastroenterol. 2015; 21:3100–3108.9. Bhardwaj A, Kedarisetty CK, Vashishtha C, et al. Carvedilol delays the progression of small oesophageal varices in patients with cirrhosis: a randomised placebo-controlled trial. Gut. 2017; 66:1838–1843.10. Bonilha DQ, Lenz L, Correia LM, et al. Propranolol associated with endoscopic band ligation reduces recurrence of esophageal varices for primary prophylaxis of variceal bleeding: a randomized-controlled trial. Eur J Gastroenterol Hepatol. 2015; 27:84–90.11. Tandon P, Abraldes JG, Keough A, et al. Risk of bacterial infection in patients with cirrhosis and acute variceal hemorrhage, based on Child-Pugh class, and effects of antibiotics. Clin Gastroenterol Hepatol. 2015; 13:1189–1196.e2.12. Azoulay D, Castaing D, Majno P, et al. Salvage transjugular intrahepatic portosystemic shunt for uncontrolled variceal bleeding in patients with decompensated cirrhosis. J Hepatol. 2001; 35:590–597.13. Puente A, Hernández-Gea V, Graupera I, et al. Drugs plus ligation to prevent rebleeding in cirrhosis: an updated systematic review. Liver Int. 2014; 34:823–833.14. Park SW, Seo YS, Lee HA, et al. Changes in cardiac varices and their clinical significance after eradication of esophageal varices by band ligation. Can J Gastroenterol Hepatol. 2016; 2016:2198163.15. Mishra SR, Sharma BC, Kumar A, Sarin SK. Primary prophylaxis of gastric variceal bleeding comparing cyanoacrylate injection and beta-blockers: a randomized controlled trial. J Hepatol. 2011; 54:1161–1167.16. Gwon DI, Kim YH, Ko GY, et al. Vascular plug-assisted retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy: a prospective multicenter study. J Vasc Interv Radiol. 2015; 26:1589–1595.17. Qiao W, Ren Y, Bai Y, Liu S, Zhang Q, Zhi F. Cyanoacrylate injection versus band ligation in the endoscopic management of acute gastric variceal bleeding: meta-analysis of randomized, controlled studies based on the PRISMA statement. Medicine (Baltimore). 2015; 94:e1725.18. Procaccini NJ, Al-Osaimi AM, Northup P, Argo C, Caldwell SH. Endoscopic cyanoacrylate versus transjugular intrahepatic portosystemic shunt for gastric variceal bleeding: a single-center U.S. analysis. Gastrointest Endosc. 2009; 70:881–887.19. Mishra SR, Chander Sharma B, Kumar A, Sarin SK. Endoscopic cyanoacrylate injection versus beta-blocker for secondary prophylaxis of gastric variceal bleed: a randomised controlled trial. Gut. 2010; 59:729–735.20. Hung HH, Chang CJ, Hou MC, et al. Efficacy of non-selective β-blockers as adjunct to endoscopic prophylactic treatment for gastric variceal bleeding: a randomized controlled trial. J Hepatol. 2012; 56:1025–1032.21. Bajaj JS, Cordoba J, Mullen KD, et al. Review article: the design of clinical trials in hepatic encephalopathy--an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther. 2011; 33:739–747.22. Lockwood AH. Blood ammonia levels and hepatic encephalopathy. Metab Brain Dis. 2004; 19:345–349.23. Tapper EB, Jiang ZG, Patwardhan VR. Refining the ammonia hypothesis: a physiology-driven approach to the treatment of hepatic encephalopathy. Mayo Clin Proc. 2015; 90:646–658.24. Kimer N, Krag A, Møller S, Bendtsen F, Gluud LL. Systematic review with meta-analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther. 2014; 40:123–132.25. Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013; 108:1458–1463.26. Als-Nielsen B, Gluud LL, Gluud C. Non-absorbable disaccharides for hepatic encephalopathy: systematic review of randomised trials. BMJ. 2004; 328:1046.27. Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010; 362:1071–1081.28. Garrido M, Turco M, Formentin C, et al. An educational tool for the prophylaxis of hepatic encephalopathy. BMJ Open Gastroenterol. 2017; 4:e000161.29. European Association for the Study of the Liver. EASL clinical practice guidelines on nutrition in chronic liver disease. J Hepatol. 2019; 70:172–193.30. Bajaj JS, Wade JB, Gibson DP, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011; 106:1646–1653.31. Jeong JY, Jun DW, Bai D, et al. Validation of a paper and pencil test battery for the diagnosis of minimal hepatic encephalopathy in Korea. J Korean Med Sci. 2017; 32:1484–1490.32. Yoon EL, Jun DW, Jeong JY, et al. Validation of the Korean Stroop test in diagnosis of minimal hepatic encephalopathy. Sci Rep. 2019; 9:8027.33. Luo M, Li L, Lu CZ, Cao WK. Clinical efficacy and safety of lactulose for minimal hepatic encephalopathy: a meta-analysis. Eur J Gastroenterol Hepatol. 2011; 23:1250–1257.34. Sidhu SS, Goyal O, Parker RA, Kishore H, Sood A. Rifaximin vs. lactulose in treatment of minimal hepatic encephalopathy. Liver Int. 2016; 36:378–385.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- KASL clinical practice guidelines for liver cirrhosis:Varices, hepatic encephalopathy, and relatedcomplications
- Current status of liver diseases in Korea: Liver cirrhosis
- Managing liver cirrhotic complications: Overview of esophageal and gastric varices
- KASL clinical practice guidelines for liver cirrhosis: Ascites and related complications
- Managements Of Liver Cirrhosis Patients In Oral And Maxillofacial Surgery -Case Reports