J Liver Cancer.
2019 Sep;19(2):108-116. 10.17998/jlc.19.2.108.
Serum PD-1 Levels Change with Immunotherapy Response but Do Not Predict Prognosis in Patients with Hepatocellular Carcinoma
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. gihankhys@yuhs.ac
- 2Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea.
- 3Yonsei Liver Center, Severance Hospital, Seoul, Korea.
- 4Biostatistics Collaboration Unit, Department of Biomedical Systems Informatics, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2463611
- DOI: http://doi.org/10.17998/jlc.19.2.108
Abstract
- BACKGROUND/AIMS
Programmed death receptor 1 (PD-1) is a promising new target for treatment of patients with hepatocellular carcinoma (HCC). A high expression level of programmed death-ligand 1 (PD-L1) is a possible prognostic indicator for poor outcome in other malignancies. Here, we investigated the clinical significance of PD-1 and PD-L1 in patients with HCC.
METHODS
We enrolled patients with HCC who underwent surgical resection at Severance Hospital between 2012 and 2017 and investigated the levels of PD-L1 in HCC tissues (tPD-L1) and PD-L1/PD-1 in serum (sPD-L1/sPD-1). We also aimed to determine whether expression levels correlated with clinical and histological features.
RESULTS
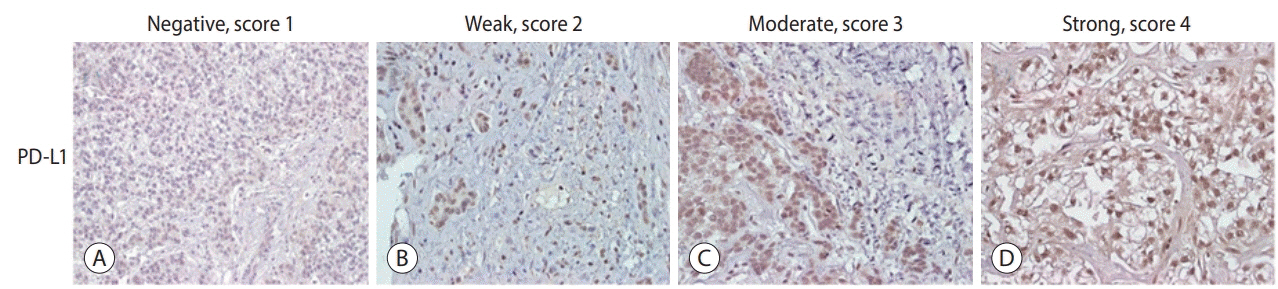
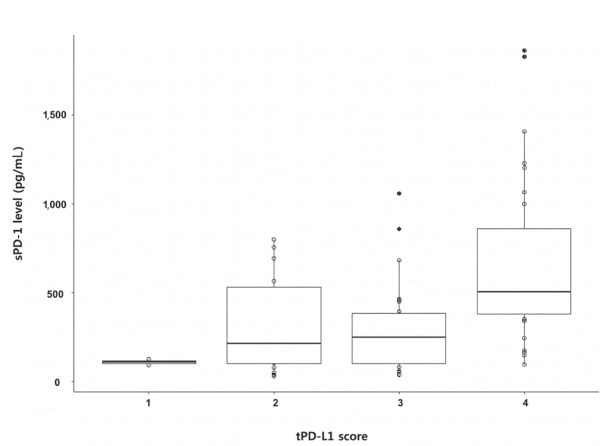
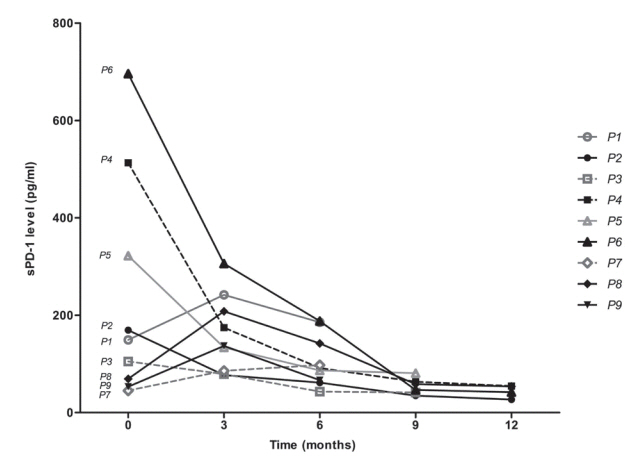
A total of 72 patient samples were analyzed. The median sPD-L1 and sPD-1 levels were 25.72 and 341.44 pg/mL, respectively. A positive correlation was detected between tPD-L1 and sPD-1 levels (R²=0.426, P<0.001). The median sPD-1 level increased linearly with tPD-L1 score (P=0.002). During the follow-up period, HCC recurred in eight (11.1%) patients and liver-related mortality occurred in eight (11.1%) patients. Higher sPD-L1 levels (≥19.18 pg/mL) tended to be associated with liver-related mortality (hazard ratio 6.866; 95% confidence interval, 0.804-58.659, P=0.078). sPD-1 levels of patients treated with nivolumab as a second-line therapy changed serially, and a >50% reduction in sPD-1 levels was observed immediately after nivolumab administration. However, sPD-1 level was not associated directly with prognosis in patients with advanced HCC.
CONCLUSIONS
The results demonstrated that PD-L1 and PD-1 levels changed according to the immunotherapy. However, no significant association with clinical outcome in patients with HCC was detected.
Figure
Cited by 2 articles
-
Current Status and Future Direction of Immunotherapy in Hepatocellular Carcinoma: What Do the Data Suggest?
Hye Won Lee, Kyung Joo Cho, Jun Yong Park
Immune Netw. 2020;20(1):e11. doi: 10.4110/in.2020.20.e11.Current Status and Future Direction of Immunotherapy in Hepatocellular Carcinoma: What Do the Data Suggest?
Hye Won Lee, Kyung Joo Cho, Jun Yong Park
Immune Netw. 2020;20(1):. doi: 10.4110/in.2020.20.e11.
Reference
-
1. Finkelmeier F, Canli Ö, Tal A, Pleli T, Trojan J, Schmidt M, et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer. 2016; 59:152–159.2. Uhercik M, Sanders AJ, Owen S, Davies EL, Sharma AK, Jiang WG, et al. Clinical significance of PD1 and PD-L1 in human breast cancer. Anticancer Res. 2017; 37:4249–4254.3. Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012; 72:917–927.4. Zheng P, Zhou Z. Human cancer immunotherapy with PD-1/PD-L1 blockade. Biomark Cancer. 2015; 7(Suppl 2):15–18.5. Zhao T, Li C, Wu Y, Li B, Zhang B. Prognostic value of PD-L1 expression in tumor infiltrating immune cells in cancers: a metaanalysis. PLoS One. 2017; 12:e0176822.6. Wang C, Zhu H, Zhou Y, Mao F, Lin Y, Pan B, et al. Prognostic value of PD-L1 in breast cancer: a meta-analysis. Breast J. 2017; 23:436–443.7. Waidmann O, Trojan J. Novel drugs in clinical development for hepatocellular carcinoma. Expert Opin Investig Drugs. 2015; 24:1075–1082.8. Disis ML. Immune regulation of cancer. J Clin Oncol. 2010; 28:4531–4538.9. Miller JF, Sadelain M. The journey from discoveries in fundamental immunology to cancer immunotherapy. Cancer Cell. 2015; 27:439–449.10. Carretero-Gonzalez A, Lora D, Ghanem I, Zugazagoitia J, Castellano D, Sepúlveda JM, et al. Analysis of response rate with ANTI PD1/PD-L1 monoclonal antibodies in advanced solid tumors: a meta-analysis of randomized clinical trials. Oncotarget. 2018; 9:8706–8715.11. Frigola X, Inman BA, Lohse CM, Krco CJ, Cheville JC, Thompson RH, et al. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011; 17:1915–1923.12. Zhou J, Mahoney KM, Giobbie-Hurder A, Zhao F, Lee S, Liao X, et al. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res. 2017; 5:480–492.13. Sayiner M, Golabi P, Younossi ZM. Disease burden of hepatocellular carcinoma: a global perspective. Dig Dis Sci. 2019; 64:910–917.14. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017; 389:2492–2502.15. Feng D, Hui X, Shi-Chun L, Yan-Hua B, Li C, Xiao-Hui L, et al. Initial experience of anti-PD1 therapy with nivolumab in advanced hepatocellular carcinoma. Oncotarget. 2017; 8:96649–96655.16. Jung HI, Jeong D, Ji S, Ahn TS, Bae SH, Chin S, et al. Overexpression of PD-L1 and PD-L2 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res Treat. 2017; 49:246–254.17. Chen CL, Pan QZ, Zhao JJ, Wang Y, Li YQ, Wang QJ, et al. PD-L1 expression as a predictive biomarker for cytokine-induced killer cell immunotherapy in patients with hepatocellular carcinoma. Oncoimmunology. 2016; 5:e1176653.18. Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asiapacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017; 11:317–370.19. Ma WJ, Wang HY, Teng LS. Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J Surg Oncol. 2013; 11:212.20. Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004; 101:796–802.21. Bilgin B, Sendur MA, Bülent Akıncı M, Şener Dede D, Yalçın B. Targeting the PD-1 pathway: a new hope for gastrointestinal cancers. Curr Med Res Opin. 2017; 33:749–759.22. Guilleminault L, Carmier D, Heuzé-Vourc'h N, Diot P, Pichon E. Immunotherapy in non-small cell lung cancer: inhibition of PD1/PDL1 pathway. Rev Pneumol Clin. 2015; 71:44–56.23. Philips GK, Atkins MB. New agents and new targets for renal cell carcinoma. Am Soc Clin Oncol Educ Book. 2014; e222–e227.24. Han Q, Wang Y, Pang M, Zhang J. STAT3-blocked whole-cell hepatoma vaccine induces cellular and humoral immune response against HCC. J Exp Clin Cancer Res. 2017; 36:156.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transcriptomic signature in advanced hepatocellular carcinoma tissue to predict combination immunotherapy response: Editorial on “Genomic biomarkers to predict response to atezolizumab plus bevacizumab immunotherapy in hepatocellular carcinoma: Insights from the IMbrave150 trial”
- Reply to correspondence on “Genomic biomarkers to predict response to atezolizumab plus bevacizumab immunotherapy in hepatocellular carcinoma: Insights from the IMbrave150 trial”
- Personalized approaches to the treatment of hepatocellular carcinoma using immune checkpoint inhibitors: Editorial on “Genomic biomarkers to predict response to atezolizumab plus bevacizumab immunotherapy in hepatocellular carcinoma: Insights from the IMbrave150 trial”
- Erratum to ‘Genomic biomarkers to predict response to atezolizumab plus bevacizumab immunotherapy in hepatocellular carcinoma: Insights from the IMbrave150 trial’ [Clin Mol Hepatol 2024;30:807-823]
- PD-L1 as a Biomarker in Gastric Cancer Immunotherapy